Dynamic Creation of a Local Acid-like Environment for Hydrogen Evolution Reaction in Natural Seawater
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Electrolysis of natural seawater driven by renewable energy is practically attractive for green hydrogen production. However, because precipitation initiated by an increase in local pH near to the cathode deactivates catalysts or blocks electrolyzer channels, limited catalysts are capable of operating with untreated, natural seawater (viz., pH 8.2 to 8.3 and ca. 35 g salts L–1); most are used in strongly alkaline or acidic seawater. Here, we report a new natural seawater electrolysis cathode with precipitation-suppression via a Pt/WO2 catalyst to create a dynamically local acid-like environment. The in situ formed hydrogen tungsten bronze (HxWOy) phase via continuous hydrogen insertion from water acts as a proton reservoir. As a result, dynamically stored protons create a local acid-like environment near the Pt active sites. We evidence that this tailored acid-like environment boosts the hydrogen evolution reaction in natural seawater splitting and neutralizes generated OH– species to restrict precipitation formations. Consequently, a long-term stability of >500 h at 100 mA cm–2 was exhibited in direct seawater electrolysis.
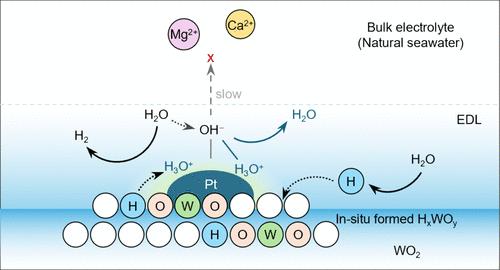
为天然海水中的氢进化反应动态创建类似酸的局部环境
利用可再生能源对天然海水进行电解,对绿色制氢具有实际吸引力。然而,由于阴极附近的局部 pH 值升高会导致沉淀,从而使催化剂失活或阻塞电解槽通道,因此能够在未经处理的天然海水(即 pH 值为 8.2 至 8.3,盐分约为 35 克/升)中运行的催化剂非常有限;大多数催化剂都用于强碱性或酸性海水中。在此,我们报告了一种新型天然海水电解阴极,它通过 Pt/WO2 催化剂抑制沉淀,以创造一种动态的局部酸性环境。通过从水中持续插入氢气而在原位形成的氢钨青铜(HxWOy)相可作为质子库。因此,动态储存的质子在铂活性位点附近创造了一种局部类酸环境。我们的证据表明,这种量身定制的类酸环境促进了天然海水分裂过程中的氢进化反应,并中和了生成的 OH- 物种,从而限制了沉淀的形成。因此,在 100 mA cm-2 的条件下,该器件在直接电解海水中表现出了 500 h 的长期稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


