Structural Disorder of a Layered Lithium Manganese Oxide Cathode Paving a Reversible Phase Transition Route toward Its Theoretical Capacity
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
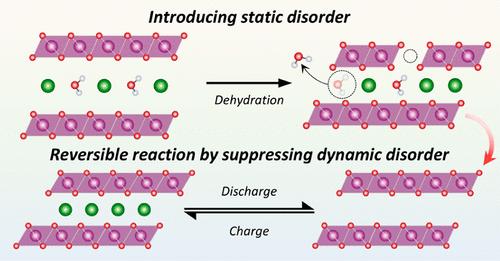
Layered lithium manganese oxides suffer from irreversible phase transitions induced by Mn migration and/or dissolution associated with the Jahn–Teller effect (JTE) of Mn3+, leading to inevitable capacity fading during cycling. The popular doping strategy of oxidizing Mn3+ to Mn4+ to relieve the JTE cannot completely eliminate the detrimental structural collapse from the cooperative JTE. Therefore, they are considered to be impractical for commercial use as cathode materials. Here, we demonstrate a layered lithium manganese oxide that can be charged and discharged without any serious structural collapse using metastable Li-birnessite with controlled structural disorder. Although Li-birnessite is thermodynamically unstable under ambient conditions, Li ion exchange into Na-birnessite followed by an optimal dehydration resulted in a disordered Li-birnessite. The control over crystal water in the interlayer provides intriguing short-range order therein, which can help to suppress parasitic Mn migration and dissolution, thereby ensuring a reversible electrochemical cycling. The Mn redox behavior and local structure change of the Li-birnessite were investigated by ex situ soft X-ray absorption spectroscopy (sXAS) and X-ray pair distribution function (PDF) analysis. The combined sXAS and PDF with electrochemical analyses disclosed that the reversible Mn redox and suppressed phase transitions in Dh Li-birnessite contribute to dramatically improving its electrochemical reversiblity during cycling. Our findings underscore the substantial effects of controlled static disorder on the structural stability and electrochemical reversibility of a layered lithium manganese oxide, Li-birnessite, which extends the practical capacity of layered oxides close to their theoretical limit.
层状氧化锰锂阴极的结构紊乱为实现理论容量铺平了一条可逆相变之路
层状锂锰氧化物受到与 Mn3+ 的 Jahn-Teller 效应 (JTE) 相关的锰迁移和/或溶解引起的不可逆相变的影响,导致在循环过程中不可避免地出现容量衰减。目前流行的掺杂策略是将 Mn3+ 氧化成 Mn4+ 以缓解 JTE,但这一策略并不能完全消除协同 JTE 带来的有害结构坍塌。因此,它们被认为无法作为正极材料投入商业应用。在这里,我们展示了一种层状锂锰氧化物,它可以利用具有可控结构紊乱的桦锂酸锂进行充电和放电,而不会出现任何严重的结构塌陷。虽然锂桦锰酸盐在环境条件下热力学不稳定,但锂离子交换到锂桦锰酸盐中,然后进行最佳脱水处理,就得到了无序的锂桦锰酸盐。对层间晶体水的控制提供了有趣的短程有序性,有助于抑制寄生锰的迁移和溶解,从而确保电化学循环的可逆性。通过原位软 X 射线吸收光谱(sXAS)和 X 射线对分布函数(PDF)分析,研究了锂桦烷石的锰氧化还原行为和局部结构变化。软 X 射线吸收光谱和 PDF 与电化学分析相结合的结果表明,锰的可逆氧化还原和 Dh 锆锂辉石中被抑制的相变有助于显著提高其在循环过程中的电化学可逆性。我们的研究结果凸显了受控静态无序对层状锂锰氧化物(锂桦烷石)的结构稳定性和电化学可逆性的重大影响,从而使层状氧化物的实际容量接近其理论极限。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


