Oxygen Vacancy Boosts Nitrogen-Centered Radical Coupling Initiated by Primary Amine Electrooxidation
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
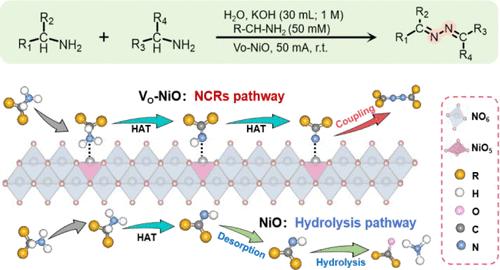
Synthesis of nitrogen-centered radicals (NCRs) for radical coupling reactions is a powerful and versatile tool in the arsenal of organic synthetic chemistry. However, there are few reports on the direct synthesis of NCRs based on aqueous electrocatalysis. Herein, we present a new electrochemical primary amine oxidation reaction (ePAOR) system with R1R2-CH-NH2 as the substrate for synthesizing NCRs and N–N coupling products. However, ePAOR on the model catalyst (NiO) suffers from low N–N coupling selectivity due to the weak adsorption energy of imine (R1R2-C═NH) intermediates. Guided by theoretical calculations, the oxygen vacancy gives NiO a strong adsorption capacity of R1R2-C═NH so that it boosts nitrogen-centered radical coupling initiated by the ePAOR on oxygen vacancy-rich NiO (VO-NiO), and the effective utilization rate of NCRs was increased from 36 to 75%. This approach is compatible with a wide range of primary amines and can be applied to N–N cross-coupling systems as well.
氧空位促进了由伯胺电氧化引发的以氮为中心的自由基耦合
合成用于自由基偶联反应的氮中心自由基(NCRs)是有机合成化学武库中一个强大而多用途的工具。然而,基于水电催化直接合成氮中心自由基的报道却很少。在此,我们提出了一种以 R1R2-CH-NH2 为底物合成 NCR 和 N-N 偶联产物的新型电化学伯胺氧化反应(ePAOR)体系。然而,由于亚胺(R1R2-C═NH)中间体的吸附能较弱,模型催化剂(NiO)上的 ePAOR 存在 N-N 偶联选择性低的问题。在理论计算的指导下,氧空位使 NiO 对 R1R2-C═NH 具有很强的吸附能力,从而提高了富氧空位 NiO(VO-NiO)上由 ePAOR 引发的氮中心自由基偶联,并将 NCR 的有效利用率从 36% 提高到 75%。这种方法与多种伯胺兼容,也可应用于 N-N 交叉偶联体系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


