lncRNAs maintain the functional phase state of nucleolar prion-like protein to facilitate rRNA processing
IF 16.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Liquid-to-solid phase transition of proteins with prion-like domains (PLDs) has been associated with neurodegenerative diseases and aging. High protein concentration is one important aspect triggering the transition; however, several prion-like proteins, including fibrillarin (FBL), an important phase-separated protein in the nucleolus for pre-rRNA processing, show relatively high expression levels in certain cells, especially cancer cells, without obvious phase transitions and growth arrest. How cells maintain prion-like protein proteostasis is still unknown. Here, we attempt to answer the question, with FBL as an example. We find that lncRNA DNAJC3-AS1 can buffer the behavior of FBL condensation and maintain the state and function of fibrillar component/dense fibrillar component (FC/DFC) units in human cell lines through two mechanisms, not only facilitating FBL condensation but also inhibiting excessive aggregation by binding multiple PLDs and partially blocking their interactions. We propose that lncRNAs could supply buffered systems to sustain functional phase states of prion-like proteins.
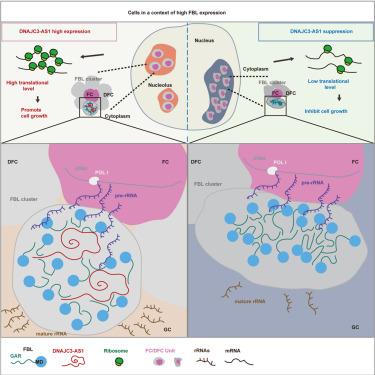
lncRNA维持核极朊病毒样蛋白的功能相态,促进rRNA加工
具有朊病毒样结构域(PLD)的蛋白质从液态到固态的相变与神经退行性疾病和衰老有关。高蛋白浓度是引发这种转变的一个重要方面;然而,包括纤丝蛋白(FBL)在内的几种朊病毒样蛋白在某些细胞,尤其是癌细胞中表现出相对较高的表达水平,但却没有明显的相转变和生长停滞。细胞如何维持朊病毒样蛋白的蛋白稳态仍是未知数。在这里,我们试图以FBL为例回答这个问题。我们发现,lncRNA DNAJC3-AS1可以通过两种机制缓冲FBL的凝聚行为,维持人细胞系中纤维成分/致密纤维成分(FC/DFC)单元的状态和功能,不仅可以促进FBL的凝聚,还可以通过结合多个PLDs并部分阻断它们之间的相互作用来抑制过度聚集。我们认为,lncRNA可提供缓冲系统,维持朊病毒样蛋白的功能相态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
文献相关原料
公司名称
产品信息
阿拉丁
NaAsO2
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


