Efficient synthesis of trisubstituted allenes via palladium-catalysed deaminative coupling of tertiary propargylamines with arylsiloxanes†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A novel and efficient method for the palladium-catalysed and highly regioselective synthesis of trisubstituted allenes via deaminative coupling of tertiary propargylamines with arylsiloxanes is reported. Both unactivated propargylamines and arylsiloxanes were activated in situ using Et3N·3HF, followed by cross-coupling to facilitate C–C bond formation through C–N bond cleavage. The method demonstrates exceptional functional group compatibility, high yields, and readily accessible coupling partners.
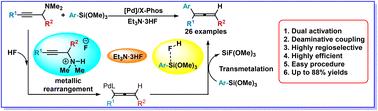

通过钯催化三级丙炔胺与芳基硅氧烷的脱氨基偶联高效合成三取代烯烃
本研究报告采用了一种新颖高效的方法,通过三级丙炔胺与芳基硅氧烷的脱氨基偶联,在钯催化下高区域选择性地合成了三取代烯烃。使用 Et3N-3HF 对未活化的丙炔胺和芳基硅氧烷进行原位活化,然后进行交叉耦合,通过 C-N 键裂解促进 C-C 键的形成。该方法具有优异的官能团兼容性、高产率和易于获得的耦合伙伴。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


