Lewis Acidic Ionic-Liquid-Catalyzed Radical-Cascade Alkylation/Cyclization of N-Alkyl-N-methacryloyl Benzamides with Alkanes via Visible-Light-Induced Ligand-to-Metal Charge Transfer: Access to Alkylated Isoquinoline-1,3(2H,4H)-diones
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
With the flourishing progress of ligand-to-metal charge transfer (LMCT) photocatalysis, various metals were developed as catalysts to activate abundant alkane feedstocks for the synthesis of functionalized organic compounds. However, to the best of our knowledge, most of the LMCT catalysts are difficult to recover and reuse for the next cycles. Herein, we report a reusable Lewis acidic ionic liquid (LAIL)-catalyzed radical-cascade alkylation/cyclization of N-alkyl-N-methacryloyl benzamides with unactivated alkanes for the synthesis of alkylated isoquinoline-1,3-(2H,4H)-diketones. The protocol features mild reaction conditions, high atom utilization efficiency, scale-up synthesis, simple operation, and recycling of catalysts.
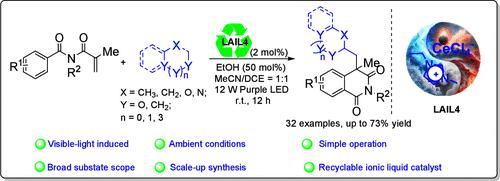
通过可见光诱导的配体-金属电荷转移催化 N-烷基-N-甲基丙烯酰基苯甲酰胺与烷烃的自由基级联烷化/环化反应:获得烷基化异喹啉-1,3(2H,4H)-二酮
随着配体-金属电荷转移(LMCT)光催化技术的蓬勃发展,各种金属被开发为催化剂,用于活化丰富的烷烃原料,合成功能化有机化合物。然而,据我们所知,大多数 LMCT 催化剂都难以回收并在下一个循环中重复使用。在此,我们报告了一种可重复使用的路易斯酸性离子液体(LAIL)催化的 N-烷基-N-甲基丙烯酰基苯甲酰胺与未活化烷烃的自由基级联烷基化/环化反应,用于合成烷基化异喹啉-1,3-(2H,4H)-二酮。该方案具有反应条件温和、原子利用效率高、可放大合成、操作简单和催化剂可回收利用等特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


