Targeting METTL3 as a checkpoint to enhance T cells for tumour immunotherapy
Abstract
Background
Immunotherapy has emerged as a crucial treatment modality for solid tumours, yet tumours often evade immune surveillance. There is an imperative to uncover novel immune regulators that can boost tumour immunogenicity and increase the efficacy of immune checkpoint blockade (ICB) therapy. Epigenetic regulators play critical roles in tumour microenvironment remodelling, and N6-methyladenosine (m6A) is known to be involved in tumourigenesis. However, the role of m6A in regulating T-cell function and enhancing anti-tumour immunity remains unexplored.
Methods
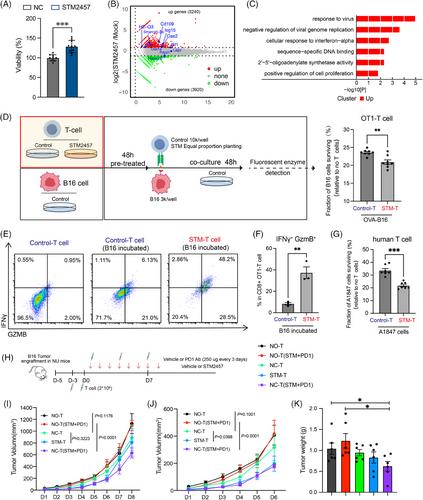
Several cancer cell lines were treated with STM2457, an enzymatic inhibitor of RNA m6A methyltransferase METTL3, and explored the transcriptome changes with RNA sequencing (RNA-seq). We then utilised mouse melanoma (B16) and mouse colorectal adenocarcinoma (MC38) models to investigate the effects of METTL3 inhibition on immunotherapy, and analysed the dynamics of the tumour microenvironment via single-cell RNA-seq (scRNA-seq). Furthermore, in vitro and in vivo T-cell cytotoxicity killing assay and CRISPR Cas9-mediated m6A reader YTHDF1-3 knockout in B16 were performed to assess the role and the molecular mechanism of RNA m6A in tumour killing. Finally, the efficacy of METTL3 inhibition was also tested on human melanoma model (A375) and human T cells.
Results
We demonstrate that inhibiting METTL3 augments tumour immunogenicity and sustains T-cell function, thereby enhancing responsiveness to ICB therapy. Mechanistically, METTL3 inhibition triggers an interferon response within tumour cells, amplifying the anti-tumour immune response, along with deletion of the m6A reader protein YTHDF2 in tumours inhibiting major histocompatibility complex (MHC)-I degradation. Remarkably, these anti-tumour effects are reliant on the immune system. Specifically, METTL3 inhibition enhances interferon-gamma (IFNγ) and granzyme B (GzmB) expression, thereby strengthening T-cell killing ability, and concurrently dampening the expression of exhaustion-related genes.
Conclusion
Targeting METTL3 enhances anti-tumour immunity by boosting T-cell cytotoxicity and reversing T-cell exhaustion. Our study positions METTL3 as an epigenetic checkpoint, highlighting the potential of targeting METTL3 to invigorate intrinsic anti-tumour defenses and overcome immune resistance.
Key points
- Targeting METTL3 augments tumour cell immunogenicity and sustains T-cell function.
- T cell with METTL3 inhibition can reverse T-cell exhaustion, and promote expression of IFNγ and GzmB, thereby enhancing cytotoxicity in anti-PD-1 therapy.
- YTHDF2 deletion in tumours prolong the lifespan of MHC-I mRNAs.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


