Kinetic Modeling Enables Understanding of Off-Cycle Processes in Pd-Catalyzed Amination of Five-Membered Heteroaryl Halides
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The mechanism of Pd-catalyzed amination of five-membered heteroaryl halides was investigated by integrating experimental kinetic analysis with kinetic modeling through predictive testing and likelihood ratio analysis, revealing an atypical productive coupling pathway and multiple off-cycle events. The GPhos-supported Pd catalyst, along with the moderate-strength base NaOTMS, was previously found to promote efficient coupling between five-membered heteroaryl halides and secondary amines. However, slight deviations from the optimal concentration, temperature, and/or solvent resulted in significantly lower yields, contrary to typical reaction optimization trends. We found that the coupling of 4-bromothiazole with piperidine proceeds through an uncommon mechanism in which the NaOTMS base, rather than the amine, binds first to the oxidative addition complex; the resulting OTMS-bound Pd species is the resting state. Formation of the Pd-amido complex via base/amine exchange was identified as the turnover-limiting step, unlike other reported catalyst systems for which reductive elimination is turnover-limiting. We determined that the amine-bound Pd complex, usually an on-cycle intermediate, is instead a reversibly generated off-cycle species, and that base-mediated decomposition of 4-bromothiazole is the primary irreversible catalyst deactivation pathway. Predictive testing and kinetic modeling were key to the identification of these off-cycle processes, providing insight into minor mechanistic pathways that are difficult to observe experimentally. Collectively, this report reveals the unique enabling features of the Pd-GPhos/NaOTMS system, implementing mechanistic insights to improve the yields of particularly challenging coupling reactions. Moreover, these findings highlight the utility of applying predictive tests to kinetic models for the rapid evaluation of mechanistic possibilities in small-molecule catalytic systems.
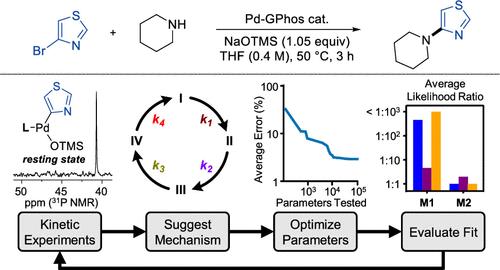
动力学建模有助于了解 Pd 催化五元杂芳基卤化物胺化过程中的非循环过程
通过预测测试和似然比分析,将实验动力学分析与动力学建模相结合,研究了钯催化五元杂芳基卤化物胺化的机理,揭示了一种非典型的生产性偶联途径和多个非循环事件。以前曾发现 GPhos 支持的钯催化剂和中等强度的碱 NaOTMS 能促进五元杂芳基卤化物和仲胺之间的高效偶联。然而,稍微偏离最佳浓度、温度和/或溶剂就会导致产率显著降低,这与典型的反应优化趋势相反。我们发现,4-溴噻唑与哌啶的偶联反应是通过一种不常见的机理进行的,在这种机理中,NaOTMS 碱而不是胺首先与氧化加成复合物结合;由此产生的与 OTMS 结合的 Pd 物种是静止状态。通过碱/胺交换形成的钯-氨基复合物被确定为限制转化率的步骤,这与其他报道的以还原消除为限制转化率的催化剂体系不同。我们确定与胺结合的钯复合物通常是周期内的中间产物,但它却是可逆生成的周期外物种,而碱介导的 4-溴噻唑分解是催化剂失活的主要不可逆途径。预测性测试和动力学建模是确定这些非循环过程的关键,为深入了解难以通过实验观察到的次要机理途径提供了依据。总之,本报告揭示了 Pd-GPhos/NaOTMS 系统的独特功能,通过对机理的深入了解,提高了特别具有挑战性的偶联反应的产率。此外,这些发现还强调了将预测性测试应用于动力学模型以快速评估小分子催化系统的机理可能性的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


