Rhodium-Catalyzed Asymmetric Reductive Hydroformylation of α-Substituted Enamides
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Chiral γ-amino alcohols are prevalent structural motifs in natural products and bioactive compounds. Nevertheless, efficient and atom-economical synthetic methods toward enantiomerically enriched γ-amino alcohols are still lacking. In this study, a highly enantioselective rhodium-catalyzed reductive hydroformylation of readily available α-substituted enamides is developed, providing a series of pharmaceutically valuable chiral 1,3-amino alcohols in good yields and excellent enantioselectivities in a single step. The development of the 4,4′-bisarylamino-substituted BIBOP ligand is crucial for the success of this transformation. DFT calculations and experimental data have revealed the importance of hydrogen bonding between the N–H group in the structure of TFPNH-BIBOP and the enamide carbonyl group in promoting both high enantioselectivity and reactivity. This method has enabled the concise synthesis of several chiral pharmaceutical intermediates including a single-step synthesis of the key chiral intermediate of maraviroc.
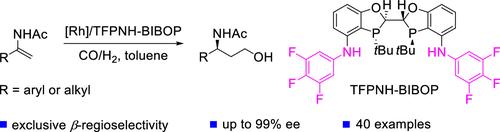
铑催化的 α-取代烯酰胺的不对称还原加氢甲酰化反应
手性γ-氨基醇是天然产物和生物活性化合物中普遍存在的结构基团。然而,目前仍缺乏高效且原子经济的合成方法来获得对映体富集的γ-氨基醇。本研究开发了一种高对映体选择性的铑催化α-取代烯酰胺还原加氢甲酰化反应,一步即可获得一系列具有医药价值的手性 1,3- 氨基醇,且产率高,对映体选择性极佳。4,4′-双芳酰胺取代的 BIBOP 配体的开发对于这一转化的成功至关重要。DFT 计算和实验数据揭示了 TFPNH-BIBOP 结构中的 N-H 基团与烯酰胺羰基之间的氢键在促进高对映体选择性和反应活性方面的重要性。该方法实现了多种手性药物中间体的简便合成,包括马拉韦罗关键手性中间体的一步合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


