Time-restricted eating reveals a “younger” immune system and reshapes the intestinal microbiome in human
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Time-restricted eating (TRE) has been shown to extent lifespans in drosophila and mouse models by affecting metabolic and anti-inflammatory activities. However, the effect of TRE on the human immune system, especially on immunosenescence, intestinal microbiome, and metabolism remains unclear. We conducted a 30-day 16:8 TRE single-arm clinical trial with 49 participants. Participants consumed daily meals from 9 a.m. to 5 p.m., provided by a nutrition canteen with a balanced, calorie-appropriate nutrition, which is designed by clinical nutritionists (ChiCTR2200058137). We monitored weight changes and weight-related parameters and focused on changes in the frequency of CD4+ senescent T cells, immune repertoire from peripheral blood, as well as serum metabolites and gut microbiota. We found that up to 95.9 % of subjects experienced sustained weight loss after TRE. The frequency of circulating senescent CD4+ T cells was decreased, while the frequency of Th1, Treg, Tfh-like, and B cells was increased. Regarding the immune repertoire, the proportions of T cell receptor alpha and beta chains were increased, whereas B cell receptor kappa and lambda chains were reduced. In addition, a reduced class switch recombination from immunoglobulin M (IgM) to immunoglobulin A (IgA) was observed. TRE upregulated the levels of anti-inflammatory and anti-aging serum metabolites named sphingosine-1-phosphate and prostaglandin-1. Additionally, several anti-inflammatory bacteria and probiotics were increased, such as Akkermansia and Rikenellaceae, and the composition of the gut microbiota tended to be “younger”. Overall, TRE showed multiple anti-aging effects, which may help humans maintain a healthy lifestyle to stay “young”. Clinical Trial Registration URL: https://www.chictr.org.cn/showproj.html?proj=159876.
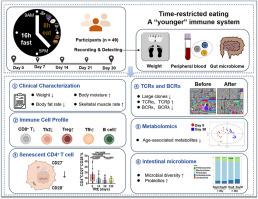
限时进食显示了 "年轻 "的免疫系统,并重塑了人类的肠道微生物群。
限时进食(TRE)通过影响新陈代谢和抗炎活动,延长了果蝇和小鼠模型的寿命。然而,限时进食对人体免疫系统的影响,尤其是对免疫衰老、肠道微生物组和新陈代谢的影响仍不清楚。我们对 49 名参与者进行了为期 30 天的 16:8 TRE 单臂临床试验。参与者每天从上午 9 点到下午 5 点进餐,由临床营养师设计的营养食堂提供均衡、热量适宜的营养(ChiCTR2200058137)。我们监测了体重变化和与体重相关的参数,并重点研究了 CD4+ 衰老 T 细胞频率、外周血免疫细胞群以及血清代谢物和肠道微生物群的变化。我们发现,多达 95.9% 的受试者在服用 TRE 后体重持续下降。循环中衰老的 CD4+ T 细胞的数量减少了,而 Th1、Treg、Tfh 样细胞和 B 细胞的数量增加了。在免疫序列方面,T细胞受体α链和β链的比例增加,而B细胞受体卡帕链和λ链的比例减少。此外,还观察到从免疫球蛋白 M(IgM)到免疫球蛋白 A(IgA)的类别转换重组减少。TRE 提高了抗炎和抗衰老血清代谢物(鞘氨醇-1-磷酸酯和前列腺素-1)的水平。此外,一些抗炎细菌和益生菌也有所增加,如 Akkermansia 和 Rikenellaceae,肠道微生物群的组成也趋于 "年轻化"。总之,TRE显示出多种抗衰老作用,可帮助人类保持健康的生活方式,从而保持 "年轻"。临床试验注册网址:https://www.chictr.org.cn/showproj.html?proj=159876。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


