Edge-substituents and center metal optimization boosting oxygen electrocatalysis in porphyrin-based covalent organic polymers
IF 9.4
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The promising non-noble electrocatalyst with well-defined structure is significant for both oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) for the renewable energy devices like zinc-air batteries (ZABs). Herein, the four phenyl-linked cobaltporphyrin-based covalent organic polymers (COPs-1–4) with the different edge substituents (1 = −tBu, 2 = −Me, 3 = −F, and 4 = −CF3) are firstly designed and synthesized via a simple, efficient one-pot method. With the increase of electron donating capacity of the substituents, the highest occupied molecular orbital energy (EHOMO) gradually increases in the order of COP-4 < COP-3 < COP-2 < COP-1. Consequently, the optimal COP-1 with −tBu edge groups exhibits the highest half-wave potential (E1/2) of 0.84 V (vs. RHE) among the four COPs, which is comparable with commercial Pt/C in alkaline media. The DFT calculations further reveal that with strong electron donating capacity, the Gibbs free energy decreases in the order of COP-4 > COP-3 > COP-2 > COP-1 by modulating the adsorption energy of OOH* at rate-determining step (RDS) to promote ORR activity. Furthermore, introducing Ni (II) and Co (II) into porphyrin centers afford the bimetallic CoNi-COP-1 with both Co-N4, Ni-N4 active sites and edge substituted −tBu. The synergistic effect of Co, Ni bimetallic active sites and strong electron-donating −tBu substituents renders the CoNi-COP-1 the highest HOMO and smallest energy gap between the ELUMO and EF among the as-prepared five COPs, which leads to more filling electrons of its LUMO level, and thus exhibits the excellent ORR and OER bifunctional catalytic activities with an E1/2 as high as 0.85 V and an overpotential (η) of 0.34 V at 10 mA cm−2 in alkaline media, superior to monometallic Co-containing COPs-1–4. In particular, the assembled ZABs with bifunctional catalyst CoNi-COP-1 possesses high power density (94.10 mW cm−2), high specific capacity (841.71 mAh gZn−1) and long durability of over 160,000 s. This work exemplifies the rational design of pyrolysis-free non-noble metal COP-based electrocatalyst through optimizing the intrinsic metal center and its secondary coordination environment.
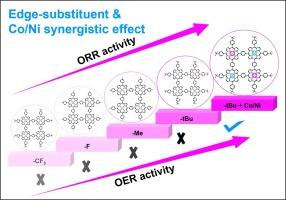
优化边缘取代基和中心金属,促进卟啉基共价有机聚合物的氧电催化。
对于锌-空气电池(ZABs)等可再生能源设备而言,具有明确结构的前景广阔的非贵金属电催化剂对于氧还原反应(ORR)和氧进化反应(OER)都具有重要意义。本文首先设计并通过简单高效的一锅法合成了四种苯基连接的钴卟啉基共价有机聚合物(COPs-1-4),它们具有不同的边缘取代基(1 = -tBu、2 = -Me、3 = -F、4 = -CF3)。随着取代基电子捐献能力的增加,四种 COPs 的最高占据分子轨道能(EHOMO)以 COP-4 1/2 的顺序逐渐增加,达到 0.84 V(相对于 RHE),与碱性介质中的商用 Pt/C 相当。DFT 计算进一步表明,在强电子捐赠能力下,通过调节速率决定步骤(RDS)中 OOH* 的吸附能,吉布斯自由能按照 COP-4 > COP-3 > COP-2 > COP-1 的顺序降低,从而促进 ORR 活性。此外,在卟啉中心引入 Ni (II) 和 Co (II) 还可产生具有 Co-N4 和 Ni-N4 活性位点以及边缘取代 -tBu 的双金属 CoNi-COP-1 。在 Co、Ni 双金属活性位点和强电子供体 -tBu 取代基的协同作用下,CoNi-COP-1 的 HOMO 最高,ELUMO 与 EF 之间的能隙最小,这使得它的 LUMO 水平有更多的填充电子,从而表现出优异的 ORR 和 OER 双功能催化活性,E1/2 高达 0.85 V,在碱性介质中 10 mA cm-2 的过电位 (η) 为 0.34 V,优于单金属含 Co COPs-1-4。这项工作体现了通过优化固有金属中心及其次级配位环境,合理设计无热解非贵金属 COP 型电催化剂的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


