The dopaminergic basis of negative symptoms in schizophrenia: an addendum
IF 9.6
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0

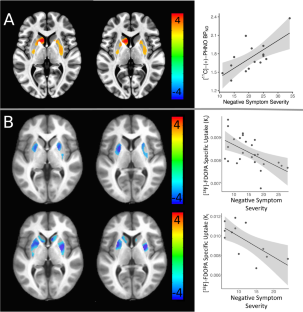
精神分裂症阴性症状的多巴胺能基础:补遗
我们想谈谈最近两项正电子发射断层扫描(PET)成像研究的结果,并结合我们自己的研究结果进行讨论[1]。我们要提及的第一项研究是在两组独立的无药精神分裂症患者中进行的[18F]FDOPA PET 调查[2]。[18F]FDOPA是多巴胺的直接前体,一般认为其摄取量反映了突触前多巴胺的合成和储存能力。与早期的[18F]FDOPA PET 研究不同,Eisenberg 等人未能发现精神分裂症患者的[18F]FDOPA 摄取量升高。不过,该研究在两组患者中都观察到了普特门的[18F]FDOPA摄取率与阴性症状严重程度之间的反相关性。我们要讨论的第二项研究[3]介绍了一项 PET 研究的结果,该研究探讨了口服哌醋甲酯(MPH)对多巴胺 D2/3 受体激动剂放射性配体[11C]-(+)-PHNO 的不可置换结合电位(BPND)值的影响。放射配体与多巴胺 D2/3 受体的结合在药理或行为挑战后发生的变化可间接测量细胞外多巴胺水平的波动。作者的目的是复制和扩展有关 CHR 患者皮层下多巴胺可用性改变的研究结果,因为之前的研究表明,在全面爆发的精神病中皮层下多巴胺功能会升高,甚至在精神病发作之前就可能存在多巴胺传递增强的现象 [4,5]。然而,最近的一项荟萃分析对此提出了质疑[6]。Girgis 等人[3]研究的主要发现是,与非慢性阻塞性肺疾病对照组相比,慢性阻塞性肺疾病受试者在接受 MPH 挑战时,[11C]-(+)-PHNO BPND 值(∆BPND)的变化更大。这与早先对精神分裂症患者进行的挑战研究结果非常吻合[1, 4, 7,8,9,10,11] ,并将这种方法的使用范围扩大到了精神病的前驱阶段(当然,这只能在事后被称为前驱阶段)。此外,Girgis 等人的研究观察到阴性症状的表现与 CHR 受试者腹侧纹状体中 [11C]-(+)-PHNO ∆BPND 之间存在反比关系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


