Macrophage NLRP3 inflammasome mediates the effects of sympathetic nerve on cardiac remodeling in obese rats
IF 3.8
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
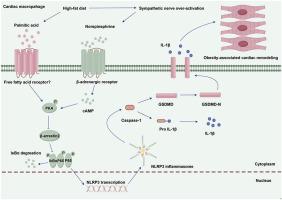
Obesity-associated cardiac remodeling is characterized by cardiac sympathetic nerve over-activation and pro-inflammatory macrophage infiltration. We identified norepinephrine (NE), a sympathetic neurotransmitter, as a pro-inflammatory effector to activate macrophage NLRP3 inflammasome, which contributed to cardiac inflammation. In vivo, Sprague-Dawley (SD) rats were fed a high-fat diet (HFD) for 12 weeks to establish obese rat models. Obese rats exhibited marked cardiac hypertrophy compared to normal rats. The expression of NLRP3 and interleukin (IL)-1β was upregulated, accompanied by CD68+NLRP3+ macrophage infiltration in the hearts of the obese rats. The obese rats also showed increased sympathetic nerve activity. β-adrenergic receptor (AR) inhibition mitigated these changes. In vitro, sympathetic neurotransmitter NE significantly exacerbated palmitic acid (PA)-induced macrophage polarization toward pro-inflammatory type and NLRP3 inflammasome activation in THP-1 macrophages. It was further found that the pro-inflammatory role of NE is dependent on the activation of protein kinase A (PKA) and subsequently inhibition of β-arrestin2, which is an important regulator of the nuclear factor-kappa B (NF-κB) pathway.
This study identifies the neuro-immune axis as an important mediator in obesity-associated cardiac remodeling. Targeting the neuro-immune system may open therapeutic opportunities for the treatment of cardiac remodeling in obesity.
巨噬细胞 NLRP3 炎性体介导交感神经对肥胖大鼠心脏重塑的影响
肥胖相关性心脏重塑的特点是心脏交感神经过度激活和促炎性巨噬细胞浸润。我们发现交感神经递质去甲肾上腺素(NE)是一种促炎效应物质,可激活巨噬细胞 NLRP3 炎性体,从而导致心脏炎症。在体内,Sprague-Dawley(SD)大鼠被喂食高脂肪饮食(HFD)12周,以建立肥胖大鼠模型。与正常大鼠相比,肥胖大鼠表现出明显的心脏肥大。肥胖大鼠心脏中的 NLRP3 和白细胞介素(IL)-1β 表达上调,并伴有 CD68+NLRP3+ 巨噬细胞浸润。肥胖大鼠的交感神经活动也有所增加。β-肾上腺素能受体(AR)抑制减轻了这些变化。在体外,交感神经递质 NE 显著加剧了棕榈酸(PA)诱导的 THP-1 巨噬细胞向促炎型极化和 NLRP3 炎性体的激活。研究进一步发现,NE 的促炎作用依赖于激活蛋白激酶 A(PKA),进而抑制核因子-卡巴 B(NF-κB)通路的重要调节因子 β-arrestin2。这项研究发现,神经免疫轴是肥胖相关性心脏重塑的重要介质。以神经免疫系统为靶点可能为治疗肥胖症的心脏重塑带来治疗机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


