Methylation cytometric pretreatment blood immune profiles with tumor mutation burden as prognostic indicators for survival outcomes in head and neck cancer patients on anti-PD-1 therapy
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Tissue biomarkers for immune checkpoint inhibitor (ICI) response are limited by tumor sample heterogeneity and availability. This study identifies clinically actionable pretreatment blood biomarkers that are associated with ICI treatment response and survival in recurrent/metastatic head and neck squamous cell carcinoma. A prospective multi-center study enrolled 100 patients before standard-of-care immunotherapy. Blood immune profiles, measured by methylation cytometry, were assessed alongside tumor mutational burden (TMB) and PD-L1 combined proportion score (CPS). TMB and PD-L1 CPS were available for 56 and 91 patients, respectively. High neutrophils, monocytes, and neutrophil-to-lymphocyte ratio were associated with worse survival, while high CD4T cells, especially naïve CD4T cells, and lymphocyte-to-monocyte ratio were associated with better survival. Significant interactions between TMB and peripheral immune profiles for both progression-free and overall survival were found. Clinically relevant pretreatment peripheral immune biomarkers were identified, demonstrating the potential of DNA-based immune profiling to predict ICI response before treatment.
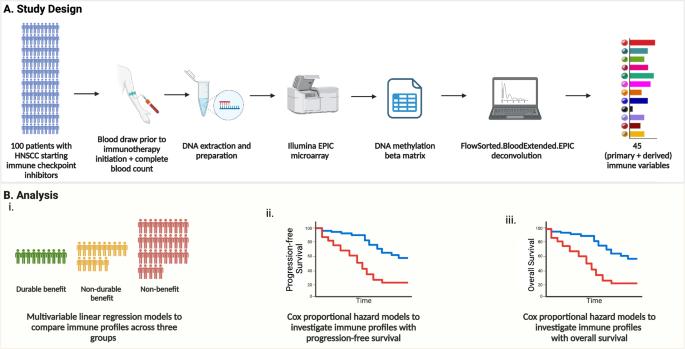
甲基化细胞学预处理血液免疫图谱与肿瘤突变负荷作为头颈癌患者接受抗PD-1治疗后生存结果的预后指标。
免疫检查点抑制剂(ICI)反应的组织生物标志物受到肿瘤样本异质性和可用性的限制。本研究确定了与复发/转移性头颈部鳞状细胞癌 ICI 治疗反应和生存相关的临床可操作的预处理血液生物标志物。一项前瞻性多中心研究招募了 100 名接受标准免疫疗法的患者。通过甲基化细胞仪测量的血液免疫概况与肿瘤突变负荷(TMB)和PD-L1综合比例评分(CPS)一起进行了评估。分别有56名和91名患者获得了TMB和PD-L1 CPS。中性粒细胞、单核细胞和中性粒细胞与淋巴细胞比值高与生存率降低有关,而CD4T细胞(尤其是幼稚CD4T细胞)和淋巴细胞与单核细胞比值高与生存率提高有关。TMB和外周免疫特征与无进展生存期和总生存期之间存在显著的交互作用。研究发现了与临床相关的治疗前外周免疫生物标志物,证明了基于DNA的免疫图谱分析在治疗前预测ICI反应的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


