Catalytic Asymmetric Cycloaddition of Olefins with In Situ Generated N-Boc-Formaldimine.
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Chiral 1,3-amino alcohols are ubiquitous structural motifs in natural products and active pharmaceutical ingredients. We present a highly enantioselective, inverse-electron-demand hetero-Diels-Alder reaction of olefins with in situ generated N-Boc-formaldimine catalyzed by strong and confined Bro̷nsted acids. This transformation provides direct access to valuable 1,3-amino alcohols from styrenes and 1,1-disubtituted alkenes. Isotope labeling studies and kinetic analysis reveal an unusual mechanism involving an oxazinium intermediate and a catalyst order greater than one.
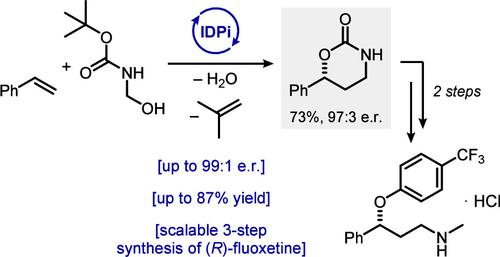
烯烃与原位生成的 N-Boc-Formaldimine 的催化不对称环加成。
手性 1,3-氨基醇是天然产品和活性药物成分中无处不在的结构基团。我们介绍了在强约束 Bro̷nsted 酸催化下,烯烃与原位生成的 N-Boc-formaldimine 发生的高对映选择性、反电子需求的异 Diels-Alder 反应。这种转化可以直接从苯乙烯和 1,1- 二亚硝基烯烃中获得有价值的 1,3- 氨基醇。同位素标记研究和动力学分析揭示了一种不寻常的机理,其中涉及一个草铵中间体和一个大于 1 的催化剂阶数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


