Exploring rotational isomerism of fluorescent diarylethenes comprising 2-naphthyl and regioisomeric oxazoyl groups. A combined experimental and computational study
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
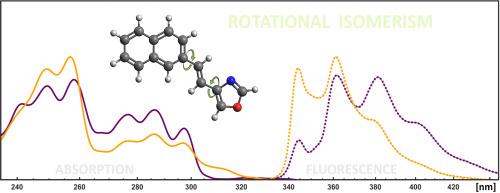
In the paper a phenomenon of rotational (conformational) isomerism within 1,2-diarylethenic compounds (DAE) comprising heteroarene ring of oxazole is explored on the example of the fluorescent naphthyl-vinyl-oxazole framework. In this regard three regioisomers of a n-(2-(naphthalen-2-yl)vinyl)oxazole molecule (nNVOx), differing in the oxazole substitution pattern (n = 2, 4, 5), are synthesized and methodically investigated by means of electronic UV–Vis spectroscopy combined with chemometric methods for spectral data analysis. Design of the experiments and interpretation of their output is additionally supported by quantum-chemical simulations based on the (TD) DFT methodology. As the result, all the studied nNVOx compounds are unveiled to simultaneously occur in form of at least two non-equivalent rotational isomers, varying in the conformation taken by the arene moieties with respect to the central vinyl fragment. The individual rotamers are then demonstrated to exhibit non-identical spectroscopic properties expressed by differences in corresponding absorption and fluorescence profiles, thereby ultimately confirming the impact of rotamerism onto the investigated molecular systems.
探索包含 2-萘基和草酰基的 Regioisomeric diarylethenes 的荧光旋转异构体。实验与计算相结合的研究
本文以荧光萘基乙烯基噁唑框架为例,探讨了包含噁唑杂环的 1,2-二芳基化合物(DAE)中的旋转(构象)异构现象。为此,我们合成了正(2-(萘-2-基)乙烯基)噁唑分子(nNVOx)的三种区域异构体,它们在噁唑取代模式(n = 2、4、5)上各不相同,并通过电子紫外可见光谱结合光谱数据分析的化学计量学方法进行了研究。此外,基于 (TD) DFT 方法的量子化学模拟也为实验设计和结果解释提供了支持。结果揭示出,所有研究的 nNVOx 化合物都同时存在至少两种非等价的旋转异构体,炔基相对于中心乙烯基片段的构象各不相同。这些旋转异构体通过相应的吸收和荧光曲线的差异表现出非相同的光谱特性,从而最终证实了旋转异构体对所研究分子体系的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


