Synthesis, DFT investigation, molecular docking analysis, ADMET studies, and biological evaluation of a novel series of imidazolidinone derivatives as potential antimicrobial agents
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
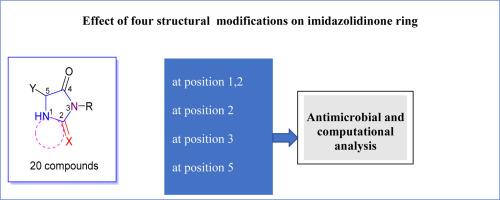
In this study, we constructed twenty novel imidazolidinone derivatives via the reaction of 2-(methylthio)-3,5-dihydro-4H-imidazol-4-one derivatives (1a-c) with some active methylene reagents and nitrogen nucleophiles. The synthesized compounds were confirmed through spectral analysis such as 1H NMR, 13C NMR, FT-IR, and MS. Moreover, the synthesized compounds were optimized and utilizing the DFT/B3LYP/6–31(G) basis set to investigate their energies and the presence of two forms of isomers (E and Z). The results confirmed the stability of the E form. ADMET of new imidazolidinones was also studied to investigate their lipophilicity and Lipinski's rule for determination of their physiological biological analysis. Also, the antimicrobial activity of new compounds on Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, Streptococcus mutans, Candida albicans, and Aspergillus Nigar using the inhibition zone technique were evaluated. The results demonstrate that compound 11c showed higher activity rather than other compounds due to the presence of piperazine moiety out of the plane of the benzene ring. Additionally, the docking study showed an electrostatic bonding interaction of the hydrogen of 11c and the amino acids of two proteins such as PDBID: 3t88 and 2wje.
作为潜在抗菌剂的一系列新型咪唑烷酮衍生物的合成、DFT 研究、分子对接分析、ADMET 研究和生物学评价
在本研究中,我们通过 2-(甲硫基)-3,5-二氢-4H-咪唑-4-酮衍生物 (1a-c) 与一些活性亚甲基试剂和氮亲核物的反应,构建了 20 种新型咪唑烷酮衍生物。合成的化合物通过 1H NMR、13C NMR、FT-IR 和 MS 等光谱分析得到了证实。此外,还利用 DFT/B3LYP/6-31(G) 基集对合成的化合物进行了优化,以研究其能量和两种异构体形式(E 和 Z)的存在。结果证实了 E 形式的稳定性。还研究了新咪唑烷酮的 ADMET,以调查它们的亲脂性和利宾斯基法则,从而确定它们的生理生物分析。此外,还采用抑菌区技术评估了新化合物对大肠杆菌、肺炎克雷伯氏菌、金黄色葡萄球菌、变异链球菌、白色念珠菌和黑曲霉的抗菌活性。结果表明,由于化合物 11c 中的哌嗪分子位于苯环平面之外,因此其活性高于其他化合物。此外,对接研究显示 11c 的氢与两种蛋白质(如 PDBID:3t88 和 2wje)的氨基酸之间存在静电键相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


