Novel 4-((3-fluorobenzyl)oxy)benzohydrazide derivatives as promising anti-prostate cancer agents: Synthesis, characterization and in vitro & in silico biological activity studies
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this study, ten novel halogenated arylidenehydrazide derivatives were synthesized and characterized through ¹H, ¹³C APT, ¹⁹F NMR, HSQC, HMBC, HRMS, and FT-IR techniques. Cytotoxic evaluations against PC3 prostate cancer and HUVEC cell lines identified compounds 8 and 14 as lead candidates, achieving IC₅₀ values of 4.49 µM and 4.78 µM, respectively, with notable selectivity indexes of 12.15 and 11.78, underscoring their specificity against PC3 cells. Molecular docking studies targeting AR, VEGFR1, EGFR, and VEGFR2 suggested potential inhibitory mechanisms, with compounds 8 and 14 displaying substantial binding affinities for AR and VEGFR1. Compound 8 achieved IFD scores of -12.900 kcal/mol for AR and -10.895 kcal/mol for VEGFR1, while compound 14 recorded scores of -10.323 kcal/mol and -10.379 kcal/mol, respectively. Complementary MM-GBSA analyses revealed favorable binding energies, with compound 8 yielding ΔG values of -76.60 kcal/mol (AR) and -78.08 kcal/mol (VEGFR1) and compound 14 showing -80.67 kcal/mol (AR) and -78.61 kcal/mol (VEGFR1). MD simulations confirmed complex stability over 50 ns, indicating that compound 14 exhibited enhanced binding stability with key residues in AR and VEGFR1. ADME predictions highlighted drug-like properties, particularly for compounds 8 and 14, with high lipophilicity and favorable absorption characteristics, despite low aqueous solubility. SAR analysis emphasized the beneficial impact of halogen substitutions on potency and selectivity, establishing compounds 8 and 14 as promising candidates for further therapeutic development.
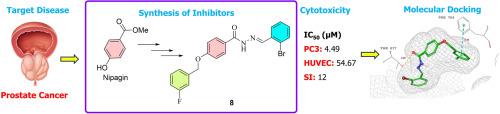
新型 4-((3-氟苄基)氧基)苯并酰肼衍生物作为有前途的抗前列腺癌药物:合成、表征、体外和硅学生物活性研究
本研究合成了十种新型卤代芳基肼衍生物,并通过¹H、¹³C APT、¹⁹F NMR、HSQC、HMBC、HRMS 和 FT-IR 技术对其进行了表征。通过对 PC3 前列腺癌细胞系和 HUVEC 细胞系进行细胞毒性评估,确定化合物 8 和 14 为主要候选化合物,其 IC₅₀ 值分别为 4.49 µM 和 4.78 µM,选择性指数分别为 12.15 和 11.78,突出了它们对 PC3 细胞的特异性。针对 AR、血管内皮生长因子受体 1、表皮生长因子受体和血管内皮生长因子受体 2 的分子对接研究表明了潜在的抑制机制,化合物 8 和 14 对 AR 和血管内皮生长因子受体 1 具有很强的结合亲和力。化合物 8 与 AR 的 IFD 得分为 -12.900 kcal/mol,与 VEGFR1 的 IFD 得分为 -10.895 kcal/mol,而化合物 14 的得分分别为 -10.323 kcal/mol 和 -10.379 kcal/mol。互补 MM-GBSA 分析显示了有利的结合能,化合物 8 的 ΔG 值为 -76.60 kcal/mol (AR) 和 -78.08 kcal/mol (VEGFR1),化合物 14 显示为 -80.67 kcal/mol (AR) 和 -78.61 kcal/mol (VEGFR1)。MD 模拟证实复合物稳定性超过 50 ns,表明化合物 14 与 AR 和 VEGFR1 中的关键残基的结合稳定性增强。ADME 预测强调了类似药物的特性,尤其是化合物 8 和 14,尽管水溶性较低,但具有高亲脂性和良好的吸收特性。SAR 分析强调了卤素取代对药效和选择性的有利影响,从而将化合物 8 和 14 确定为有希望进一步开发治疗药物的候选化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


