A framework for robust glaucoma detection: A confidence-aware deep uncertainty quantification approach with a comprehensive assessment for enhanced clinical decision-making
IF 7.5
2区 计算机科学
Q1 AUTOMATION & CONTROL SYSTEMS
Engineering Applications of Artificial Intelligence
Pub Date : 2024-11-13
DOI:10.1016/j.engappai.2024.109651
引用次数: 0
Abstract
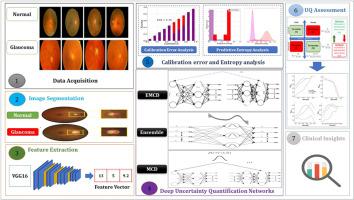
Glaucoma poses a significant threat to public health worldwide, as it can result in irreversible vision loss. Timely identification is vital for halting the progression of visual field deterioration. In recent years, deep neural networks (DNNs) have become increasingly popular in medical imaging due to their ability to identify patterns. As a result, this study introduces a new computer-aided diagnosis (CAD) system based on deep learning (DL) algorithms for glaucoma detection that extracts meaningful features from retinal fundus images (RFIs) and employs uncertainty quantification (UQ) models, including Monte Carlo dropout (MCD), ensemble Bayesian, and ensemble Monte Carlo dropout (EMCD), to generate both point estimates and confidence values for the outputs, thereby capturing the uncertainty associated with the classifications. The proposed framework is validated using well-known clinical datasets, and the reliability of the outputs is evaluated using comprehensive performance metrics such as expected calibration error (ECE), entropy analysis, and a multi-criteria UQ assessment. Experimental results demonstrate the superiority of the ensemble model, with uncertainty accuracies registering at 97.64%, 97.26%, and 98.97% for the “ACRIMA”, “RIM-ONE-DL”, and “ORIGA” datasets, respectively. Moreover, the proposed algorithms can alert users to the majority of erroneous diagnoses by assigning uncertainty labels, providing valuable insights for clinicians in glaucoma detection. Such tools can assist healthcare professionals in reducing the probability of misdiagnosis and ensuring that patients receive timely and appropriate treatment.
稳健青光眼检测框架:可信度感知的深度不确定性量化方法与综合评估,促进临床决策
青光眼可导致不可逆转的视力丧失,对全球公众健康构成重大威胁。及时识别对于阻止视野恶化的进展至关重要。近年来,深度神经网络(DNN)凭借其识别模式的能力在医学成像领域越来越受欢迎。因此,本研究介绍了一种基于深度学习(DL)算法的新型计算机辅助诊断(CAD)系统,该系统用于青光眼检测,可从视网膜眼底图像(RFIs)中提取有意义的特征,并采用不确定性量化(UQ)模型,包括蒙特卡罗剔除(MCD)、集合贝叶斯(Bayesian)和集合蒙特卡罗剔除(EMCD)模型,为输出结果生成点估计和置信度值,从而捕捉与分类相关的不确定性。利用著名的临床数据集对所提出的框架进行了验证,并利用预期校准误差 (ECE)、熵分析和多标准 UQ 评估等综合性能指标对输出的可靠性进行了评估。实验结果表明了集合模型的优越性,"ACRIMA"、"RIM-ONE-DL "和 "ORIGA "数据集的不确定性准确率分别为 97.64%、97.26% 和 98.97%。此外,所提出的算法还能通过分配不确定性标签,提醒用户注意大多数错误诊断,为临床医生检测青光眼提供有价值的见解。这些工具可以帮助医护人员降低误诊概率,确保患者得到及时、适当的治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Engineering Applications of Artificial Intelligence
工程技术-工程:电子与电气
CiteScore
9.60
自引率
10.00%
发文量
505
审稿时长
68 days
期刊介绍:
Artificial Intelligence (AI) is pivotal in driving the fourth industrial revolution, witnessing remarkable advancements across various machine learning methodologies. AI techniques have become indispensable tools for practicing engineers, enabling them to tackle previously insurmountable challenges. Engineering Applications of Artificial Intelligence serves as a global platform for the swift dissemination of research elucidating the practical application of AI methods across all engineering disciplines. Submitted papers are expected to present novel aspects of AI utilized in real-world engineering applications, validated using publicly available datasets to ensure the replicability of research outcomes. Join us in exploring the transformative potential of AI in engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


