Influence of 2,1,3-pyrazinochalcogenadiazoles structure on their dimerization via chalcogen bonding (chalcogen = S, Se, Te)
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Chalcogen bonding (ChB) is an σ-hole-driven secondary bonding interaction (SBI). The crystalline 2,1,3-benzochalcogenadiazoles involved in organic optoelectronics is exemplified by [E···N]2 supramolecular synthon. For 5,6- R2 −2,1,3-pyrazinochalcogenadiazoles E-M and [E···N]2-bonded (E-M)2 (E = S, Se, Te; R/M = H/1, Me/2, CN/3), gas-phase and dichloromethane solution calculations are performed. The molecular electrostatic potential suggests that changes in E, R influence σ- and π-holes of E-M/(E-M)2. Distant R acts via long-range electrostatic field effect. ChB strength increases in the order S < Se < Te, and (E-2)2 < (E-1)2 < (E-3)2. The main driving forces are electrostatic and dispersion interactions. Crystalline S-1 and Se-2 have head-to-head dimers. Se-3 shows head-to-tail chains via Se···Ncyano ChB. A competition between different ChB, and, between ChB and other SBIs, should be considered in the design and synthesis of new E-M/(E-M)2 for fundamentals and applications.
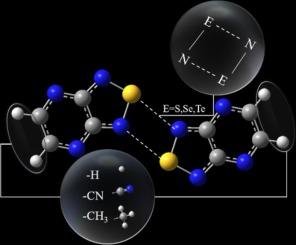
2,1,3-吡嗪基胆原二唑结构对其通过胆原键(胆原 = S、Se、Te)进行二聚化的影响
钙原键(ChB)是一种由σ-孔驱动的次键相互作用(SBI)。有机光电子学中涉及的 2,1,3-苯并羰基噻唑结晶是[E--N]2 超分子合成的典范。研究人员对 5,6- R2 -2,1,3-吡嗪基喹二唑 E-M 和 [E---N]2 键合 (E-M)2 (E = S、Se、Te;R/M = H/1、Me/2、CN/3)进行了气相和二氯甲烷溶液计算。分子静电势表明,E、R 的变化会影响 E-M/(E-M)2 的 σ 孔和 π 孔。远距离 R 通过长程静电场效应起作用。ChB 强度按 S < Se < Te 和 (E-2)2 < (E-1)2 < (E-3)2 的顺序增加。主要的驱动力是静电和分散相互作用。晶体 S-1 和 Se-2 具有头对头二聚体。Se-3 通过 Se-Ncyano ChB 显示头尾链。在设计和合成用于基本原理和应用的新型 E-M/(E-M)2时,应考虑不同 ChB 之间以及 ChB 与其他 SBI 之间的竞争。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


