Halogen substitution effects in Chlorostannate(II) hybrid material: Insights from DFT study on 2(C4H4FN3O)·SnX6·2(H2O), (X = F, Cl, Br, I)
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The development of new materials is crucial for advancing technologies in material science, energy, and biology. In this study, we investigate the effect of Halogen-substitution on the compound 2(C4H4FN3O)·SnX6·2(H2O) (where X = F, Cl, Br, and I). Using extensive theoretical analysis, we examined the impact of halogen substitution on the structural properties, band gap energy (Eg), and chemical behavior. Our results show that the Eg decreases with the increasing atomic number of halogens. The I-substituted compound is the most reactive and exhibits significant intramolecular charge transfer, enhancing its antioxidant properties. The spatial distribution of electron density in the Frontier Molecular Orbitals (FMOs) indicates that the HOMO is localized on the inorganic anion and the LUMO on the organic cation, a pattern consistent across all substituted compounds. These findings highlight the potential of these compounds in electron transfer reactions and provide insights for tailoring them for specific chemical and biochemical applications.
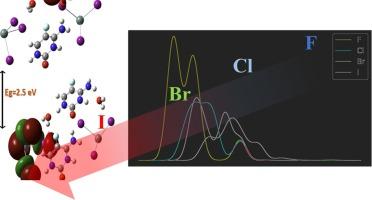
氯锡酸(II)杂化材料中的卤素取代效应:对 2(C4H4FN3O)-SnX6-2(H2O)(X = F、Cl、Br、I)的 DFT 研究的启示
开发新材料对于推动材料科学、能源和生物技术的发展至关重要。在本研究中,我们研究了卤素取代对化合物 2(C4H4FN3O)-SnX6-2(H2O)(其中 X = F、Cl、Br 和 I)的影响。通过大量的理论分析,我们研究了卤素取代对结构特性、带隙能(Eg)和化学行为的影响。结果表明,Eg 随卤素原子数的增加而降低。I 取代的化合物反应性最强,表现出显著的分子内电荷转移,从而增强了其抗氧化性。前沿分子轨道(FMO)中电子密度的空间分布表明,HOMO 位于无机阴离子上,LUMO 位于有机阳离子上,所有取代的化合物都是如此。这些发现凸显了这些化合物在电子转移反应中的潜力,并为定制它们以用于特定的化学和生物化学应用提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


