Cluster aggregation of water-based deep eutectic solvents in water and evaluation of their cytotoxicity
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Deep Eutectic Solvents (DESs) are emerging in the recent literature as promising environmentally friendly liquids in different topics, finding fruitful applications as substitutes to common volatile organic solvents.
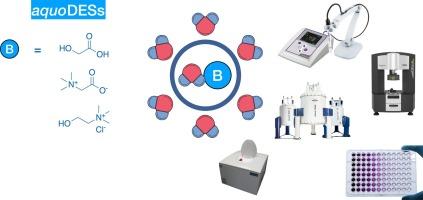
In this work a study of cluster formation of water-based DESs in water dilutions is presented. The three analysed mixtures are: glycolic acid/water (GA/H2O); betaine/water (TMG/H2O), choline chloride/water (ChCl/H2O). Ionic conductivity, viscosity, ultrasound spectroscopy and NMR techniques showed the presence of aggregates of aquoDESs in water dilutions. These aggregates are present in the solutions until values about 50 % w/w of added water, showing the presence of water-in-water structured systems. Moreover, taking into account the convenient disposal as water dilution of a DES, the cytotoxicity of these dilutions was evaluated on Caco2 model cells, showing the effect to be related only to the non-water components of these liquids. The water dilutions of water-based DESs have a great relevance for their applications, because their physical properties can be modulated as needed preserving their DESs’ identities.
水基深共晶溶剂在水中的团聚及其细胞毒性评估
深共晶溶剂(DESs)作为有前途的环境友好型液体在最近的文献中出现在不同的主题中,作为常见挥发性有机溶剂的替代品找到了富有成效的应用。分析的三种混合物是:乙醇酸/水(GA/H2O)、甜菜碱/水(TMG/H2O)和氯化胆碱/水(ChCl/H2O)。离子电导率、粘度、超声波谱和核磁共振技术表明,在水稀释液中存在含水沉积物的聚集体。这些聚集体一直存在于溶液中,直到添加水的重量百分比达到 50%左右,这表明存在水包水结构系统。此外,考虑到以水稀释的方式处理 DES 比较方便,还在 Caco2 模型细胞上对这些稀释液的细胞毒性进行了评估,结果表明这种影响只与这些液体中的非水成分有关。水基 DES 的水稀释液对其应用具有重要意义,因为它们的物理特性可以根据需要进行调节,从而保持 DES 的特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


