Predicting metastable oxide-to-hydroxide phase transformations by bulk and interface thermodynamics: Application to the phase stability of aluminium oxides and hydroxides in water
IF 8.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
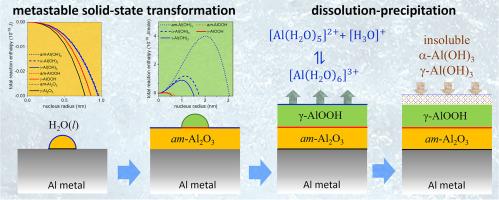
Fundamental understanding of the phase stability of oxides in aqueous environments is of eminent importance for a broad range of disciplines, such as corrosion, tribology, catalysis, medical implants, biosensing and environmental sciences. Most oxide-to-hydroxide phase transformation sequences proceed by consecutive formation of different metastable hydroxide phases towards the most stable bulk hydroxide phase, in accord with Ostwald's Rule of Stages and thus in contradiction with bulk thermodynamics. In this work, a novel unified thermodynamic model is presented for predicting such metastable oxide-to-hydroxide phase transformation sequences by accounting for the energy barrier(s) associated with the creation of new interface(s) between the competing parent and product phase(s). To this end, semi-empirical expressions for the estimation of the interface energies between metals, oxides and/or hydroxides were derived based on the macroscopic atom approach. Application of the model to the phase stability of aluminium in water (at neutral pH) for 298 ≤ T ≤ 500 K predicts a solid-state phase transformation sequence from Al → am-Al2O3 → (pseudo)boehmite, in accord with experimental observations. Subsequent competing formation of the trihydroxide phases bayerite and gibbsite by a dissolution-precipitation on the (pseudo)boehmite surface is equally favoured and critically depends on the hydrolysis of dissolved Al3+ species in solution, as governed by e.g. pH, temperature and the presence of foreign ions in solution.
通过块体热力学和界面热力学预测氧化物到氢氧化物的蜕变:应用于铝氧化物和氢氧化物在水中的相稳定性
从根本上了解氧化物在水环境中的相稳定性,对于腐蚀、摩擦学、催化、医疗植入、生物传感和环境科学等众多学科都非常重要。大多数氧化物到氢氧化物的相变顺序都是通过连续形成不同的可褪色氢氧化物相,最终形成最稳定的主体氢氧化物相,这符合奥斯特瓦尔德阶段法则,因此与主体热力学相矛盾。本研究提出了一个新颖的统一热力学模型,通过考虑在相互竞争的母相和产物相之间建立新界面所产生的能量障碍,预测这种可褪色氧化物到氢氧化物的相变顺序。为此,基于宏观原子方法,推导出了估算金属、氧化物和/或氢氧化物之间界面能量的半经验表达式。将该模型应用于 298 ≤ T ≤ 500 K 时铝在水(中性 pH 值)中的相稳定性,预测了铝 → am-Al2O3 → (伪)玻镁铁的固态相变序列,与实验观察结果一致。随后在(伪)波长石表面通过溶解-沉淀作用竞争形成的三氢氧化物相贝叶石和吉布斯特同样有利,并且关键取决于溶液中溶解的 Al3+ 物种的水解作用,例如 pH 值、温度和溶液中外来离子的存在。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Materialia
工程技术-材料科学:综合
CiteScore
16.10
自引率
8.50%
发文量
801
审稿时长
53 days
期刊介绍:
Acta Materialia serves as a platform for publishing full-length, original papers and commissioned overviews that contribute to a profound understanding of the correlation between the processing, structure, and properties of inorganic materials. The journal seeks papers with high impact potential or those that significantly propel the field forward. The scope includes the atomic and molecular arrangements, chemical and electronic structures, and microstructure of materials, focusing on their mechanical or functional behavior across all length scales, including nanostructures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


