One-pot synthesis of tricyclic pyrano[3.2-c]chromene derivatives and their evaluation through in vitro immunomodulatory activity against T cells; Molecular docking investigations and ADME-Tox prediction studies
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
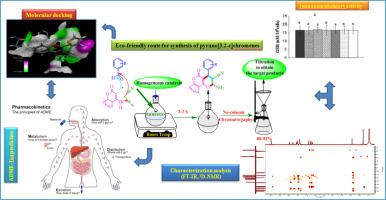
The aim of this work is the in vitro determination of the immunomodulation effect of pyrano[3,2-c]chromenes on the proliferation of T lymphocyts. These tricyclic heterocycles were prepared at room temperature via a one-pot, three-component condensation reaction involving 4‑hydroxy-2H-chromen-2-one, aromatic aldehydes, and malononitrile, using morpholine as a catalyst in a 1:1 ethanol/water mixture. This multicomponent reaction affords the product in high yields, in accordance with the criteria of green chemistry. The structure elucidation was accomplished by spectral data, IR, NMR (1H, 13C, 2D). We determined the in vitro effects of 2-amino-4-(3‑hydroxy-5-methoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (AC09) the 2-amino-5-oxo-4-(thiophen-2-yl)-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (AC11) derivatives, on the proliferative responses of human T lymphocyts cells, cytokine secretion and intracellular redox status. The study revealed that compounds AC09 and AC11 are efficient immunostimulants in a dose-dependent manner.
Human lymphocytes were less sensitive to AC11 at high concentrations compared to the potency of AC09. Human lymphocyte IL-2, IFNγ and IL-4 secretions were significantly enhanced by those pyrano[3,2-c]chromenes derivatives in a dose-dependent manner. The levels of intracellular lymphocytes glutathione (GSH), were unaffected by AC09 and AC11 at any concentrations. AC09 and AC11 induce a significant increase in hydroperoxide and carbonyl protein contents at high concentrations. Conversely, a computational study using molecular modelling revealed that compounds AC09 and AC11 exhibit a strong affinity for the active site residues of Interferon-γ (IFN-γ; PDB ID: 6E3K) target. This is confirmed by the low score energy value and the formation of different types of interactions such as: hydrogen bonds, hydrophobic interactions, and van der Waals forces. Moreover, all Drug likeness's rules were validated, and no toxicity is presented by these compounds. Finally, in vitro studies, molecular docking techniques and ADME-T (Absorption, Distribution, Metabolism, Excretion and Toxicity) evaluations were successfully conducted, aiding in the identification of new IFN-γ target inhibitors. A comparative analysis /or studies was then carried out to highlight the complementary effects of the two approaches.
三环吡喃并[3.2-c]色烯衍生物的单锅合成及其对 T 细胞的体外免疫调节活性评估;分子对接调查和 ADME-Tox 预测研究
这项工作的目的是体外测定吡喃并[3,2-c]色烯对 T 淋巴细胞增殖的免疫调节作用。这些三环杂环是在室温下,以吗啉为催化剂,在 1:1 的乙醇/水混合物中,通过涉及 4-羟基-2H-色烯-2-酮、芳香醛和丙二腈的单锅三组分缩合反应制备的。这种多组分反应的产率很高,符合绿色化学的标准。通过光谱数据、红外光谱、核磁共振(1H、13C、2D),我们完成了结构阐释。5H-吡喃并[3,2-c]苯并吡喃-3-腈(AC11)衍生物对人类 T 淋巴细胞增殖反应、细胞因子分泌和细胞内氧化还原状态的影响。研究发现,AC09 和 AC11 化合物是高效的免疫刺激剂,其作用呈剂量依赖性。与 AC09 的效力相比,人类淋巴细胞对高浓度 AC11 的敏感性较低。这些吡喃并[3,2-c]苯并吡喃衍生物以剂量依赖的方式显著提高了人类淋巴细胞IL-2、IFNγ和IL-4的分泌。细胞内淋巴细胞谷胱甘肽(GSH)的水平不受任何浓度的 AC09 和 AC11 的影响。高浓度的 AC09 和 AC11 会导致过氧化氢和羰基蛋白含量显著增加。相反,利用分子模型进行的计算研究显示,AC09 和 AC11 化合物对干扰素-γ(IFN-γ;PDB ID:6E3K)靶标的活性位点残基具有很强的亲和力。低分能值以及氢键、疏水相互作用和范德华力等不同类型相互作用的形成证实了这一点。此外,所有药物相似性规则都得到了验证,这些化合物没有毒性。最后,还成功地进行了体外研究、分子对接技术和 ADME-T(吸收、分布、代谢、排泄和毒性)评估,从而帮助确定了新的 IFN-γ 靶点抑制剂。随后进行了对比分析或研究,以突出两种方法的互补作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


