A novel porous Zn-MOF based on binuclear metal clusters for fluorescence detection of Cr(VI) and adsorption of dyes
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
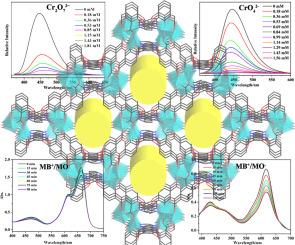
A novel Zn-MOF: {[Zn3(L)2(H2O)4]·(H2O)3·(CH3CN)3}n was synthesized from tricarboxylate pyridine ligand 3-(2,4-dicarboxyphenyl) -4-carboxypyridine (H3 L) under solvothermal conditions. Zn-MOF was a three-dimensional porous framework composed of binuclear metal cluster [Zn2(COO)3 N] and a variety of metal oxygen chains. The fluorescence study of Zn-MOF indicated that the maximum emission peak is 449 nm (λex=335 nm), and it had varying degrees of quenching effect on Cr2O72−and CrO42− in water, the limits of detection are 4.29×10−4 M and 9.19×10−5 M, respectively. It also showed good recycling ability, which was a potential multi-functional anion fluorescence probe material. In addition, the dye adsorption and separation experiments of Zn-MOF showed that it can adsorb MB+ and MG+, but almost no adsorption for MO−, and can effectively separate MB+ and MG+ from the mixed solution of MB+/MO− and MG+/MO−. It was a potential multi-functional material for the capacity to selectively separate cationic dyes from anionic and cationic mixed solutions, as well as selective recognition of Cr2O72−and CrO42−anions.
基于双核金属团簇的新型多孔 Zn-MOF,用于荧光检测六价铬和吸附染料
新型 Zn-MOF: {[Zn3(L)2(H2O)4]-(H2O)3-(CH3CN)3}n 是由三羧酸吡啶配体 3-(2,4-二羧基苯基) -4- 羧基吡啶 (H3 L) 在溶热条件下合成的。Zn-MOF 是由双核金属团簇 [Zn2(COO)3 N] 和多种金属氧链组成的三维多孔框架。对 Zn-MOF 的荧光研究表明,其最大发射峰为 449 nm(λex=335 nm),对水中的 Cr2O72 和 CrO42- 有不同程度的淬灭作用,检测限分别为 4.29×10-4 M 和 9.19×10-5 M。它还表现出良好的回收能力,是一种潜在的多功能阴离子荧光探针材料。此外,Zn-MOF 的染料吸附和分离实验表明,它能吸附 MB+和 MG+,但几乎不吸附 MO-,并能从 MB+/MO-和 MG+/MO- 混合溶液中有效分离 MB+和 MG+。它具有从阴离子和阳离子混合溶液中选择性分离阳离子染料的能力,以及选择性识别 Cr2O72 和 CrO42 阴离子的能力,是一种潜在的多功能材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


