Precise synthesis of cyclopropyl methyl ketone via proton transfer of 5-chloro-2-pentanone driven by hydrogen bonds
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The traditional synthesis methods of cyclopropyl methyl ketone (CPMK) have some disadvantages such as low yield, slow reaction rate and unclear catalytic mechanism. To solve these problems, a new strategy is developed to synthesize CPMK from 5‑chloro-2-pentanone (CPE) using 1,8-diazabicyclo[5.4.0]undec‑7-ene (DBU). Experiment results show that the yield of CPMK reached a maximum of 96.5 % in DBU at 40 °C for 30min. The mechanism study shows that the hydrogen bond between DBU and CPE is the key to drive the deprotonation and cyclization of CPE. The hydrogen bond is formed between the N atom on C = N of DBU and the α-H on the methylene group associated with the carbonyl group on CPE. The catalyst DBU is converted into [DBUH]+Cl−, which can be reclaimed and recycled completely. The density functional theory (DFT) calculation confirmed the existence of molecular hydrogen bonds and proved that the hydrogen bond driving force is the key to the reaction. The hydrogen bond driven mechanism provides a new idea for the basic research of cyclization reaction.
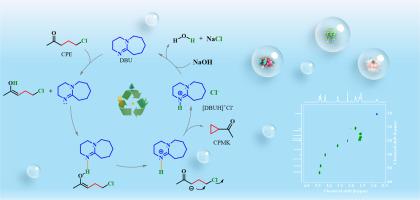
通过氢键驱动 5-Cloro-2-pentanone 质子转移精确合成环丙基甲基酮
环丙基甲基酮(CPMK)的传统合成方法存在收率低、反应速率慢、催化机理不明确等缺点。为了解决这些问题,研究人员开发了一种新策略,利用 1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)从 5-氯-2-戊酮(CPE)合成 CPMK。实验结果表明,在 DBU 中,温度为 40 °C,时间为 30 分钟,CPMK 的产率最高可达 96.5%。机理研究表明,DBU 和 CPE 之间的氢键是驱动 CPE 发生去质子化和环化反应的关键。氢键是在 DBU 的 C = N 上的 N 原子和 CPE 上与羰基相关的亚甲基上的α-H 之间形成的。催化剂 DBU 转化为[DBUH]+Cl-,可以完全回收和循环利用。密度泛函理论(DFT)计算证实了分子氢键的存在,并证明氢键驱动力是反应的关键。氢键驱动机理为环化反应的基础研究提供了新思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


