The limits of copper oxidation states from density functional theory computations: Fluoro-copper complexes, [CuFn]x, where n = 1 through 6 and x = 3+ through 5−
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
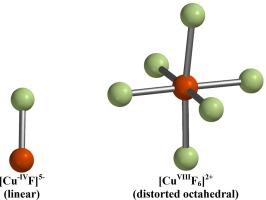
Density functional theory calculations, at the ωB97X-D/6-311+G* level of theory, were performed on homoleptic fluoro-copper complexes [CuFn]x, n = 1 through 6 and x = 3+ through 5−, to determine the highest positive and lowest negative copper oxidation states that can be supported in these complexes. Only singlet and doublet spin states were investigated. All fluoro-copper stoichiometries stabilized copper(III) or greater. However, some stoichiometries stabilized oxidation states up to copper(VI), and the greatest positive copper oxidation state was copper(VIII) in the distorted octahedral [CuF6]2+ cation. Oxidation states as negative as copper(–IV) in the diatomic [CuF]5− anion and copper(–III) in the triatomic [CuF2]5− were also observed as optimized minima, although no negative oxidation states were calculated to exist for fluoro-copper complexes containing more than two fluorine atoms. No singlet or doublet fluoro-copper complexes with charges more positive than 3+, more negative than 5−, or of Cu(VII), could be optimized.
密度泛函理论计算得出的铜氧化态极限:氟铜络合物 [CuFn]x,其中 n = 1 至 6,x = 3+ 至 5-
在 ωB97X-D/6-311+G* 理论水平上,对 n = 1 至 6 和 x = 3+ 至 5- 的同色氟铜络合物 [CuFn]x 进行了密度泛函理论计算,以确定这些络合物中可支持的最高正铜氧化态和最低负铜氧化态。只研究了单旋态和双旋态。所有氟铜化学计量学方法都能稳定铜(III)或更高。然而,有些化学结构能稳定铜(VI)以下的氧化态,而铜的最大正氧化态是畸变八面体 [CuF6]2+ 阳离子中的铜(VIII)。在二原子[CuF]5-阴离子中的铜(-IV)负氧化态和三原子[CuF2]5-中的铜(-III)负氧化态也被观测到为优化的最小值,尽管计算得出含有两个以上氟原子的氟铜络合物不存在负氧化态。电荷大于 3+、大于 5- 或 Cu(VII)的单质或双质氟铜络合物都无法优化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


