Novel s-triazine derivatives as potential anticancer agents: Synthesis, DFT, DNA binding, molecular docking, MD simulation and in silico ADMET profiling
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
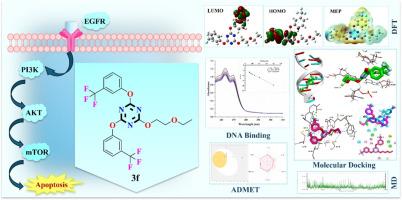
The ongoing challenge of cancer in modern medicine highlights the urgent need for targeted therapeutic strategies, particularly against the EGFR/PI3K/AKT/mTOR signalling pathways, which are pivotal in tumorigenesis. This study presents the design, synthesis, characterization, DNA binding studies and computational evaluation of a novel series of trisubstituted s-triazine derivatives 3a–n as potential anticancer agents. The synthetic methodology utilized the reaction of various oxygen-containing nucleophiles with cyanuric chloride via nucleophilic aromatic substitution, yielding the corresponding target products with high yield and purity. Density Functional Theory (DFT) calculations utilizing the B3LYP level provided insights into the electronic properties of the compounds, with derivatives 3f, 3n, and 3l displaying the lowest energy gaps and highest electrophilicity indices, indicative of their enhanced reactivity. DNA binding results from UV–Vis absorption spectroscopy confirmed the groove mode of binding for the most potent compound 3f with the binding constant (Kb) value of 6.75 × 104 M−1. Molecular docking studies against DNA (PDB ID: 3EY0) and EGFR (PDB ID: 6V6O) revealed strong binding affinities, with compound 3f (R1= ethoxy, R2= 3-CF3) exhibiting a notable binding energy of -8.9 kcal/mol and −9.1 kcal/mol respectively. Additionally, these compounds showed significant binding interactions with PI3K (PDB ID: 5HJB) and mTOR (PDB ID: 4JSV), suggesting their potential as dual inhibitors of the PI3K/AKT/mTOR pathway. The drug-likeness and ADMET profiles further support the therapeutic promise of compound 3f bearing a 3-trifluoromethyl substituent.
作为潜在抗癌剂的新型 s-三嗪衍生物:合成、DFT、DNA 结合、分子对接、MD 模拟和硅学 ADMET 分析
现代医学对癌症的持续挑战凸显了对靶向治疗策略的迫切需求,尤其是针对表皮生长因子受体/PI3K/AKT/mTOR 信号通路的靶向治疗策略。本研究介绍了一系列新型三取代 s-三嗪衍生物 3a-n 作为潜在抗癌剂的设计、合成、表征、DNA 结合研究和计算评估。合成方法是利用各种含氧亲核物与三聚氯氰通过亲核芳香取代反应,以高产率和高纯度得到相应的目标产物。利用 B3LYP 水平进行的密度泛函理论(DFT)计算深入揭示了这些化合物的电子特性,其中衍生物 3f、3n 和 3l 显示出最低的能隙和最高的亲电指数,表明它们的反应活性得到了增强。紫外可见吸收光谱的 DNA 结合结果证实了最强化合物 3f 的沟槽结合模式,其结合常数 (Kb) 值为 6.75 × 104 M-1。针对 DNA(PDB ID:3EY0)和表皮生长因子受体(EGFR)(PDB ID:6V6O)的分子对接研究显示了很强的结合亲和力,化合物 3f(R1= 乙氧基,R2= 3-CF3)的显著结合能分别为 -8.9 kcal/mol 和 -9.1 kcal/mol。此外,这些化合物还与 PI3K(PDB ID:5HJB)和 mTOR(PDB ID:4JSV)发生了显著的结合相互作用,表明它们有可能成为 PI3K/AKT/mTOR 通路的双重抑制剂。药物相似性和 ADMET 特征进一步支持了带有 3-三氟甲基取代基的化合物 3f 的治疗前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


