Synthesis and characterization of 2-(3,4-dihydroxyphenyl)-4-((4-hydroxyphenyl)imino)-4h-chromene-3,5,7-triol: In vitro biological evaluation and computational analysis
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Secondary phenolic metabolites like flavonoids, that are found in numerous plant sections and have a range of uses. Quercetin falls under the category of flavonols, belonging to the flavonoids found in various medicinal plants, some common fruits, and vegetables with various applications. However, quercetin, with its various applications, is not well established in the pharmaceutical industry since it is associated with a lot of drawbacks, like instability in the gastrointestinal tract, the first-pass effect, solubility issues, etc. Quercetin-Schiff base derivatives are essential for targeting cancer cells and enhancing biological activities due to the formation of the C=N (imine group), which plays a major role in enhancing biological activities. In this research article, quercetin Schiff base (Q1sb) was synthesised and analysed using spectroscopic techniques like 1H NMR, 13C NMR, HRMS, etc. Biological activities like anti-bacterial, antioxidant, anti-inflammatory, and cytotoxicity analyses were done on four cancer cell lines, namely A549, HeLa, HepG2, and MCF-7, and molecular docking were done on some of the common targets. The first section explains the characteristics of flavonoid, the therapeutic action and drawbacks of quercetin, and the importance of Schiff base derivatives; the second section explains the synthesis method and characterization; and the third section explains the biological applications and molecular docking studies.
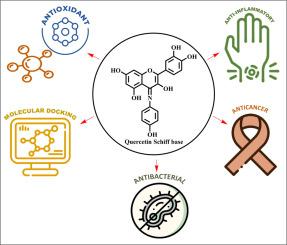
2-(3,4-二羟基苯基)-4-((4-羟基苯基)亚氨基)-4-苯并吡喃-3,5,7-三醇的合成与表征:体外生物学评价和计算分析
黄酮类等次生酚类代谢物存在于多种植物中,具有多种用途。槲皮素属于黄酮醇类,属于在各种药用植物、一些常见水果和蔬菜中发现的黄酮类化合物,具有各种用途。然而,由于槲皮素在胃肠道中的不稳定性、首过效应、溶解性问题等诸多弊端,槲皮素的各种应用在制药业中并不十分成熟。槲皮素-希夫碱衍生物在靶向癌细胞和增强生物活性方面至关重要,因为其形成的 C=N(亚胺基)在增强生物活性方面起着重要作用。本研究文章合成了槲皮素席夫碱(Q1sb),并利用 1H NMR、13C NMR、HRMS 等光谱技术对其进行了分析。在四种癌细胞系(即 A549、HeLa、HepG2 和 MCF-7)上进行了抗菌、抗氧化、抗炎和细胞毒性等生物活性分析,并对一些常见靶标进行了分子对接。第一部分介绍了黄酮类化合物的特点、槲皮素的治疗作用和缺点以及希夫碱衍生物的重要性;第二部分介绍了合成方法和表征;第三部分介绍了生物应用和分子对接研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


