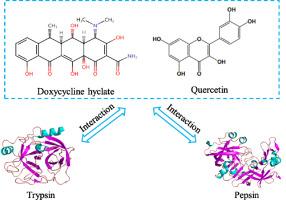
Study on the interaction mechanism of trypsin/pepsin-doxycycline hyclate-quercetin ternary system
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The interaction mechanism of trypsin/pepsin-doxycycline hyclate-quercetin ternary system was investigated by the molecular docking, molecular dynamics simulation and multiple spectroscopic methods. The results show that when doxycycline hyclate and quercetin are bound with trypsin/pepsin in sequence to form the ternary system, the different sequence of doxycycline hyclate/quercetin binding has different effects on the binding affinity of the two drugs and trypsin/pepsin. However, the binding site of the two drugs on trypsin/pepsin is basically the same as that of the corresponding binary system, except for pepsin-doxycycline hyclate (first)-quercetin ternary system. For the trypsin-quercetin (first)-doxycycline hyclate ternary system, the activity of trypsin is inhibited due to the presence of quercetin. The doxycycline hyclate that binding first even affects the binding site of quercetin and the inhibition of pepsin activity. When doxycycline hyclate and quercetin bind with trypsin/pepsin at the same time, the binding site of doxycycline hyclate and quercetin on trypsin/pepsin is completely different from their corresponding binary system. The values of binding constant of 104 L mol−1 indicate that the binding affinity are all moderate. In ternary system, the free concentration of doxycycline hyclate and quercetin in trypsin/pepsin is higher than that in binary system. Hydrophobic interactions, electrostatic forces and hydrogen bonds are the main non-covalent forces. The extent of unfolding of trypsin/pepsin peptide backbone in ternary system is greater than that in binary system. The effects of binary and ternary systems on the secondary structure of trypsin/pepsin are not significantly different, and both results in the reduction of trypsin/pepsin β-sheet.
胰蛋白酶/胃蛋白酶-多西环素-槲皮素三元体系的相互作用机制研究
通过分子对接、分子动力学模拟和多种光谱方法研究了胰蛋白酶/胃蛋白酶-土霉素强力霉素-槲皮素三元体系的相互作用机理。结果表明,当强力霉素和槲皮素依次与胰蛋白酶/胃蛋白酶结合形成三元体系时,强力霉素/槲皮素结合的不同顺序对两种药物与胰蛋白酶/胃蛋白酶的结合亲和力有不同的影响。但是,除了胃蛋白酶-强力霉素-槲皮素三元体系外,两种药物与胰蛋白酶/胃蛋白酶的结合位点与相应的二元体系基本相同。在胰蛋白酶-槲皮素(原)-环酸多西环素三元体系中,由于槲皮素的存在,胰蛋白酶的活性受到抑制。首先结合的环酸多西环素甚至会影响槲皮素的结合位点和对胃蛋白酶活性的抑制。当多西环素和槲皮素同时与胰蛋白酶/胃蛋白酶结合时,多西环素和槲皮素在胰蛋白酶/胃蛋白酶上的结合位点与相应的二元体系完全不同。其结合常数为 104 L mol-1,表明其结合亲和力均为中等。在三元体系中,土霉素和槲皮素在胰蛋白酶/胃蛋白酶中的游离浓度高于二元体系。疏水作用力、静电力和氢键是主要的非共价作用力。三元体系中胰蛋白酶/胃蛋白酶肽骨的展开程度大于二元体系。二元和三元体系对胰蛋白酶/胃蛋白酶二级结构的影响没有显著差异,都会导致胰蛋白酶/胃蛋白酶β片的减少。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


