Differentiating the Aβ42 aggregation states via intrinsic tyrosine fluorescence spectrum
IF 2.8
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aggregation of amyloid β-peptide (Aβ) into β-sheet-rich fibrils is central to the development of Alzheimer’s disease, with Aβ42 more prone to aggregation over Aβ40. Using the intrinsic tyrosine fluorescence spectrum, we show that Aβ42 exhibits a biphasic fluorescence pattern featuring a broad band and a narrow one, distinct from Aβ40 and dissolved tyrosine. Molecular dynamics simulations highlighted the differences in tyrosine’s rotamer populations and dynamics between dissolved and aggregated amyloids. Fibrillar Aβ42 shows slower, more uniform tyrosine rotations, corresponding to the narrower fluorescence band. This approach offers a rapid means to differentiate Aβ42 aggregates, benefiting Aβ-related research.
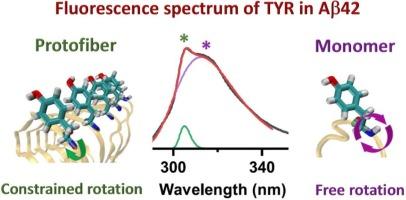
通过内在酪氨酸荧光光谱区分 Aβ42 聚合状态
淀粉样β肽(Aβ)聚集成富含β片的纤维是阿尔茨海默病发病的核心原因,其中Aβ42比Aβ40更容易聚集。我们利用固有的酪氨酸荧光光谱显示,Aβ42表现出一种双相荧光模式,具有宽带和窄带,不同于Aβ40和溶解的酪氨酸。分子动力学模拟突显了溶解淀粉样蛋白和聚集淀粉样蛋白中酪氨酸旋转体数量和动态的差异。纤维状 Aβ42 显示出更慢、更均匀的酪氨酸旋转,与更窄的荧光带相对应。这种方法提供了一种快速区分 Aβ42 聚集体的方法,有利于 Aβ 相关研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


