Boosting the phosphorus uptake of La2(CO3)3·8H2O based adsorbents via sodium addition: Relationship between crystal structure and adsorption capacity
IF 10.5
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Excess phosphate contents in water bodies triggers eutrophication, which posts significant challenges to the aquatic ecosystem. Lanthanum-carbonate based adsorbents exhibit excellent phosphate binding properties for remediating eutrophication. However, they suffer from significant adsorption-capacity loss (>85 %) at high pH. Little has been done on understanding this behavior for improving the phosphorus adsorption of lanthanum-carbonate adsorbents in alkaline environments (e.g. eutrophic water bodies). Here, we discover that La2(CO3)3·8H2O, when produced by a conversion reaction from NaLa(CO3)2·xH2O, exhibits high phosphate adsorption capacity in a wide pH window. Under alkaline conditions (e.g. pH = 10), its adsorption capacity decreases by only 8 % compared to the value under neutral pH. By isolating three different lanthanum-carbonate based compounds and analyzing their molecular structures, we find that the trace amount of Na+ residual in our La2(CO3)3·8H2O alters the chemical environment surrounding the La3+ ions, which may significantly boost the phosphate uptake at high pH. Our results provide molecular insights for further tuning the material structure of phosphate adsorbents to achieve robust performances.
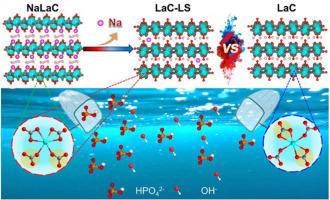
通过添加钠提高基于 La2(CO3)3-8H2O 的吸附剂对磷的吸收:晶体结构与吸附容量之间的关系
水体中磷酸盐含量过高会引发富营养化,给水生生态系统带来巨大挑战。以碳酸镧为基础的吸附剂具有出色的磷酸盐结合特性,可用于解决富营养化问题。然而,在 pH 值较高时,它们的吸附容量会明显下降(85%)。为了改善碳酸镧吸附剂在碱性环境(如富营养化水体)中对磷的吸附,人们对这种行为的了解还很少。在这里,我们发现,由 NaLa(CO3)2-xH2O 通过转化反应生成的 La2(CO3)3-8H2O 在较宽的 pH 值范围内具有较高的磷酸盐吸附能力。在碱性条件下(如 pH = 10),其吸附能力仅比中性 pH 值低 8%。通过分离三种不同的碳酸镧化合物并分析其分子结构,我们发现 La2(CO3)3-8H2O 中残留的微量 Na+ 改变了 La3+ 离子周围的化学环境,这可能会显著提高高 pH 值下的磷酸盐吸收能力。我们的研究结果为进一步调整磷酸盐吸附剂的材料结构以实现强大性能提供了分子见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Progress in Solid State Chemistry
化学-无机化学与核化学
CiteScore
14.10
自引率
3.30%
发文量
12
期刊介绍:
Progress in Solid State Chemistry offers critical reviews and specialized articles written by leading experts in the field, providing a comprehensive view of solid-state chemistry. It addresses the challenge of dispersed literature by offering up-to-date assessments of research progress and recent developments. Emphasis is placed on the relationship between physical properties and structural chemistry, particularly imperfections like vacancies and dislocations. The reviews published in Progress in Solid State Chemistry emphasize critical evaluation of the field, along with indications of current problems and future directions. Papers are not intended to be bibliographic in nature but rather to inform a broad range of readers in an inherently multidisciplinary field by providing expert treatises oriented both towards specialists in different areas of the solid state and towards nonspecialists. The authorship is international, and the subject matter will be of interest to chemists, materials scientists, physicists, metallurgists, crystallographers, ceramists, and engineers interested in the solid state.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


