Clinical Outcomes of Maintenance Durvalumab After Definitive Concurrent Chemoradiotherapy in Unresectable Locally Advanced Stage III NSCLC According to EGFR and ALK Status: Korean Cancer Study Group LU-22-18
IF 3
Q2 ONCOLOGY
引用次数: 0
Abstract
Introduction
The role of maintenance durvalumab after definitive concurrent chemoradiotherapy (CCRT) in unresectable locally advanced NSCLC with EGFR mutation or ALK translocation remains unclear. We compared the effectiveness of durvalumab maintenance therapy in groups with EGFR and ALK wild-type versus those with EGFR or ALK mutations.
Methods
In this retrospective multicenter observational study, patients with locally advanced NSCLC without progression after CCRT followed by maintenance durvalumab and available molecular test results (EGFR and ALK) were eligible. The primary objective was to compare progression-free survival (PFS) between EGFR and ALK wild-type and EGFR or ALK mutant NSCLC. Secondary objectives include overall survival according to EGFR or ALK mutation and programmed death-ligand 1 (PD-L1) expression.
Results
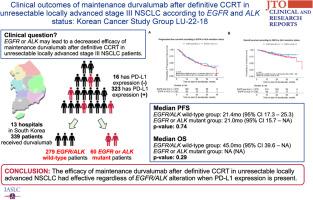
Among 339 patients, 279 had wild-type EGFR/ALK, 41 had EGFR mutations and 19 had ALK translocations. The median age was 68 years with 276 male individuals (81.4%) and 63 female individuals (18.6%), 165 (49.3%) had adenocarcinoma, 149 (44.5%) had squamous cell carcinoma, and 21 (6.3%) had other histologic types, 120 (35.4%) had stage IIIA, 168 (49.6%) stage IIIB, and 51 (15.0%) had stage IIIC. Most of the patients (n = 288, 85%) achieved partial response to CCRT, two (0.6%) had a complete response, and 49 patients (14.4%) had stable disease. Excluding four patients with unknown PD-L1 tumor proportion score (TPS), 16 (4.8%) had a PD-L1 TPS of 0, 168 (50.1%) had 1 to 49, and 151 (45.1%) had 50 or higher. The median PFS was 21.4 months (95% confidence interval [CI]: 17.3–25.3) for the EGFR/ALK wild-type group and 21.0 months (95% CI: 15.7–not available [NA]) for the EGFR or ALK mutant group with no difference (p = 0.74). Significant differences occurred in PFS on the basis of PD-L1 expression with values of 13.6 (95% CI: 10.5–NA), 18.7 (95% CI: 15.1–26.9), and 24.7 (95% CI: 20.7–NA) months for TPS of 0, 1–49, and 50 or higher, respectively (p = 0.02).
Conclusions
Durvalumab maintenance therapy after definitive CCRT in unresectable locally advanced NSCLC patients with EGFR or ALK mutation demonstrates comparable clinical outcomes to those with wild-type EGFR/ALK when PD-L1 expression is present.
根据表皮生长因子受体(EGFR)和表皮生长因子受体(ALK)状态对不可切除的局部晚期 III 期 NSCLC 进行明确的同期化疗后维持 Durvalumab 的临床疗效:韩国癌症研究小组 LU-22-18
导言:对于表皮生长因子受体(EGFR)突变或ALK易位的不可切除局部晚期NSCLC患者,在确定性同期化放疗(CCRT)后维持达伐单抗治疗的作用仍不明确。我们比较了表皮生长因子受体(EGFR)和ALK野生型组与表皮生长因子受体(EGFR)或ALK突变组中杜伐单抗维持治疗的有效性。方法在这项回顾性多中心观察性研究中,符合条件的局部晚期NSCLC患者在CCRT后接受杜伐单抗维持治疗且未出现进展,并有分子检测结果(EGFR和ALK)。首要目标是比较表皮生长因子受体(EGFR)和ALK野生型与表皮生长因子受体(EGFR)或ALK突变型NSCLC的无进展生存期(PFS)。次要目标包括根据表皮生长因子受体(EGFR)或ALK突变和程序性死亡配体1(PD-L1)表达情况确定的总生存期。结果339例患者中,279例为表皮生长因子受体(EGFR)/ALK野生型,41例为表皮生长因子受体(EGFR)突变,19例为ALK易位。中位年龄为68岁,其中男性276人(81.4%),女性63人(18.6%);165人(49.3%)为腺癌,149人(44.5%)为鳞癌,21人(6.3%)为其他组织学类型;120人(35.4%)为IIIA期,168人(49.6%)为IIIB期,51人(15.0%)为IIIC期。大多数患者(288 人,85%)对 CCRT 有部分反应,2 人(0.6%)有完全反应,49 人(14.4%)病情稳定。除去4名PD-L1肿瘤比例评分(TPS)未知的患者,16名患者(4.8%)的PD-L1肿瘤比例评分为0,168名患者(50.1%)的PD-L1肿瘤比例评分为1-49,151名患者(45.1%)的PD-L1肿瘤比例评分为50或以上。EGFR/ALK 野生型组的中位 PFS 为 21.4 个月(95% 置信区间 [CI]:17.3-25.3),EGFR 或 ALK 突变组的中位 PFS 为 21.0 个月(95% 置信区间 [CI]:15.7-not available [NA]),无差异(P = 0.74)。根据PD-L1表达情况,PFS出现显著差异,TPS为0、1-49和50或更高时,PFS分别为13.6个月(95% CI:10.5-NA)、18.7个月(95% CI:15.1-26.9)和24.7个月(95% CI:20.7-NA)(p = 0.02)。结论对于有表皮生长因子受体(EGFR)或ALK突变的不可切除局部晚期NSCLC患者,在PD-L1表达存在的情况下,确诊CCRT后的Durvalumab维持治疗可获得与野生型EGFR/ALK患者相当的临床疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


