Exceptional photocatalytic performance of hexagonal ZnO nanorods for anionic and cationic dyes degradation
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
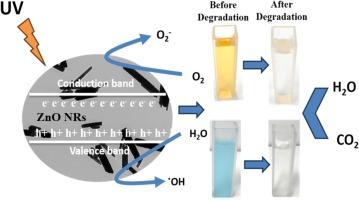
This study presents an in-depth investigation of zinc oxide hexagonal nanorods (ZnO NRs) synthesized via a hydrothermal approach at three pH basic values conducted in high-pressure laboratory reactor provided by Parr Instrument Company. The research evaluates the catalytic properties of the ZnO NRs, highlighting their potential for environmental applications. The as-fabricated samples are characterized by various techniques including the X-ray diffraction (XRD), UV–visible spectroscopy, Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), Brunauer–Emmett–Teller (BET) analysis, Field emission-scanning electron microscopy (FE-SEM), energy dispersive X-ray analysis (EDAX) and high-resolution transmission electron microscopy (HR-TEM). Rietveld refinement of X-ray diffraction data reveals the formation of high-purity hexagonal Wurtzite-type ZnO phase. Further, it is investigated that by decreasing the pH values, the grain size of ZnO NRs increases from 25.70 nm to 29.91 nm. SEM analyses further confirmed the hexagonal nanorod-shaped morphology of ZnO. The photocatalytic degradation performance of the as-fabricated ZnO NRs for Methylene Blue (MB) and Methyl Orange (MO) dyes increased with the increase in pH value, reaching almost 95 % and 64 %, respectively, after 30 min of UV irradiation. The optimum degradation is achieved at a pH value of 11.
六方氧化锌纳米棒在降解阴离子和阳离子染料方面的卓越光催化性能
本研究深入探讨了在帕尔仪器公司提供的高压实验室反应器中,通过水热法在三种 pH 基本值条件下合成的氧化锌六方纳米棒(ZnO NRs)。研究评估了 ZnO NRs 的催化特性,突出了其在环境应用方面的潜力。对制备好的样品采用了多种技术进行表征,包括 X 射线衍射 (XRD)、紫外-可见光谱、拉曼光谱、X 射线光电子能谱 (XPS)、布鲁诺-艾美特-泰勒 (BET) 分析、场发射扫描电子显微镜 (FE-SEM)、能量色散 X 射线分析 (EDAX) 和高分辨率透射电子显微镜 (HR-TEM)。对 X 射线衍射数据进行里特维尔德细化后,发现形成了高纯度的六方钨酸锌相。此外,研究还发现,随着 pH 值的降低,ZnO NRs 的晶粒尺寸从 25.70 nm 增加到 29.91 nm。扫描电镜分析进一步证实了氧化锌的六角纳米棒状形态。所制备的 ZnO NRs 对亚甲蓝(MB)和甲基橙(MO)染料的光催化降解性能随着 pH 值的增加而提高,在紫外线照射 30 分钟后分别达到近 95% 和 64%。当 pH 值为 11 时,降解效果最佳。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


