Synthesis, crystal structure and sulphide ion sensing study of a Cu(II) complex of aroyl hydrazone
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Herein, we report a Cu(II) complex {[CuII(HL)(H2O)2]Cl} of a benzoylhydrazone Schiff base ligand (4-((2-benzoylhydrazineylidene)methyl)-3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-1-ium chloride) (H2L). The molar conductance in solution, magnetic susceptibility, ESI-MS and EPR spectroscopy have been performed to justify the structure of the complex. The solid-state structure of the complex has also been solved by single crystal X-ray diffractometry. Cu(II) centre is penta-coordinated with imine-N, phenoxide-O and deprotonated amide-O donor of the ligand and two H2O molecules occupying the coordination sphere forming a distorted square pyramidal geometry (τ = 0.32). We have studied the anion sensing property of the complex by monitoring the changes in the UV–Vis and fluorescence spectra of the complex in aqueous Tris-HCl buffer medium with incremental addition of various anions as tetrabutylammonium/sodium salts. The complex shows appreciable sensitivity toward sulphide ion, among various other anions, with association constant (Kb) for binding of the complex with S2− and lowest detection limit (L.O.D) value of 6.87 × 104 M−1 and 6.9 × 10−7 M, respectively. Thus, the complex can be used as an efficient S2− probe. The mechanism of sensing is found to be displacement of the fluorescent ligand from the Cu(II) complex by the sulphide ion.
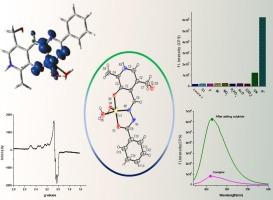
甲酰腙的 Cu(II) 复合物的合成、晶体结构和硫离子传感研究
在此,我们报告了苯甲酰肼席夫碱配体(4-((2-苯甲酰肼亚基)甲基)-3-羟基-5-(羟甲基)-2-甲基吡啶-1-氯化铵)(H2L)的铜(II)配合物{[CuII(HL)(H2O)2]Cl}。对该复合物进行了溶液摩尔电导、磁感应强度、ESI-MS 和 EPR 光谱分析,以证明其结构的合理性。此外,还通过单晶 X 射线衍射仪解决了该复合物的固态结构问题。Cu(II) 中心与配体的亚胺-N、氧化酚-O 和去质子化的酰胺-O 给体呈五配位,两个 H2O 分子占据配位层,形成扭曲的正方金字塔几何结构 (τ = 0.32)。我们研究了该配合物的阴离子传感特性,方法是在水性 Tris-HCl 缓冲介质中,以四丁基铵盐/钠盐的形式逐步加入各种阴离子,监测配合物紫外可见光谱和荧光光谱的变化。该复合物对硫离子和其他各种阴离子都非常敏感,与 S2- 结合的关联常数(Kb)和最低检测限(L.O.D)值分别为 6.87 × 104 M-1 和 6.9 × 10-7 M。因此,该复合物可用作高效的 S2- 探针。感应机制是硫离子将荧光配体从 Cu(II) 复合物中置换出来。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


