NFATc1 in CD4+ T cells and CD11c+ dendritic cells drives TH2-mediated eosinophilic inflammation in allergic asthma
The journal of allergy and clinical immunology. Global
Pub Date : 2024-10-18
DOI:10.1016/j.jacig.2024.100355
引用次数: 0
Abstract
Background
Asthma, a chronic lung disease, is a significant public health problem worldwide. It is marked by increased TH2 response resulting in eosinophil accumulation. The pathophysiology of asthma involves various cell types, including epithelial cells, dendritic cells (DCs), innate lymphoid cells, B cells, and effector cells. Nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), a critical transcription factor for immune regulation, is known for its role in T cells and, more recently, in myeloid cells. However, the specific contributions of NFATc1 in T cells and DCs in the context of asthma are not well understood.
Objective
We explored NFATc1’s role in T cells and DCs in modulating TH2 immune responses within the pathophysiology of allergic asthma.
Methods
We induced asthma in mice lacking Nfatc1 in CD4+ T cells or CD11c+ DCs using house dust mite, thereby enabling investigation into NFATc1’s role in both cell types in experimental allergic asthma. Additionally, we examined NFATc1 expression in these cell types and its correlation with blood eosinophil levels in an adult asthma cohort.
Results
In a house dust mite–induced asthma model, we found that Nfatc1 deficiency either in CD4+ T cells or CD11c+ DCs resulted in reduced TH2-driven eosinophilic inflammation, IgE levels, and mast cell presence in the lung of asthmatic mice. Nfatc1’s absence in CD4+ T cells directly hampered TH2 cell polarization and functionality, whereas in CD11c+ DCs, it affected DC differentiation and maturation, thereby weakening T-cell priming, proliferation, and subsequent TH2 differentiation. Correspondingly, translational research indicated significant correlations between CD4+NFATc1+ and CD11c+NFATc1+ cell populations and eosinophil levels in asthmatic patients, but not in healthy controls.
Conclusion
NFATc1 in T cells and DCs modulates TH2-mediated eosinophilic inflammation in allergic asthma, thus offering insight into asthma pathogenesis and identifying NFATc1 as a potential target for therapeutic intervention.
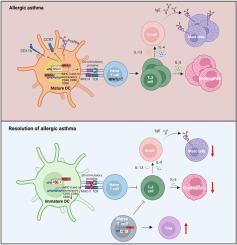
CD4+ T 细胞和 CD11c+ 树突状细胞中的 NFATc1 驱动过敏性哮喘中 TH2 介导的嗜酸性粒细胞炎症
背景哮喘是一种慢性肺部疾病,是全球重大的公共卫生问题。其特征是 TH2 反应增强,导致嗜酸性粒细胞聚集。哮喘的病理生理学涉及多种细胞类型,包括上皮细胞、树突状细胞(DC)、先天性淋巴细胞、B 细胞和效应细胞。活化 T 细胞核因子胞质 1(NFATc1)是免疫调节的一个关键转录因子,它在 T 细胞中的作用众所周知,最近又在髓系细胞中发挥了作用。我们探讨了 NFATc1 在 T 细胞和 DCs 中调节过敏性哮喘病理生理学中 TH2 免疫反应的作用。方法 我们利用屋尘螨诱导 CD4+ T 细胞或 CD11c+ DCs 中缺乏 Nfatc1 的小鼠发生哮喘,从而研究了 NFATc1 在实验性过敏性哮喘的两种细胞类型中的作用。结果在屋尘螨诱导的哮喘模型中,我们发现 CD4+ T 细胞或 CD11c+ DCs 中 Nfatc1 的缺乏会导致 TH2 驱动的嗜酸性粒细胞炎症、IgE 水平和哮喘小鼠肺中肥大细胞的存在减少。Nfatc1 在 CD4+ T 细胞中的缺失直接阻碍了 TH2 细胞的极化和功能,而在 CD11c+ DCs 中,它影响了 DC 的分化和成熟,从而削弱了 T 细胞的引诱、增殖和随后的 TH2 分化。结论T细胞和DC中的NFATc1可调节过敏性哮喘中TH2介导的嗜酸性粒细胞炎症,从而揭示哮喘的发病机制,并将NFATc1确定为治疗干预的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The journal of allergy and clinical immunology. Global
Immunology, Allergology and Rheumatology
CiteScore
0.70
自引率
0.00%
发文量
0
审稿时长
92 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


