Solvent optimization for caffeine and tannic acid extraction from Guarana seed: Insights from molecular dynamics simulations
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Guarana seeds are a rich source of bioactive compounds, particularly caffeine and tannic acid, which have significant applications in the food and pharmaceutical industries. To extract these valuable compounds from the guarana seeds, solvent extraction methods are used. Molecular dynamics (MD) simulations are employed to investigate the interaction of Guarana seed extract with three different solvents namely ethanol, ethyl acetate and Choline chloride-glycerol as deep eutectic solvent (DES) varying the molar ratio. The bonded and non-bonded interactions are computed to perform the MD simulations. The optimized potentials for liquid simulations force field are used to compute these interactions. The study examines the Radial Distribution Function (RDF) and hydrogen bonds to elucidate structural arrangement and intermolecular interactions respectively. RDF reveals that the interaction of caffeine with ethanol is strongest hence it is the best suitable solvent for its extraction. Mean Square Displacement analysis offers insights into transport properties, particularly diffusion of molecules in solvents. Primarily subdiffusive nature for the mobility of functional moieties of Guarana seed components are observed in solvents. The comparison of diffusive regimes further reveals the preferential extraction of caffeine and tannic acid over the minor compounds using ethanol. The selective separation leads their essential applications in the food industry.
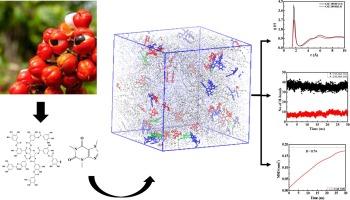
从瓜拉那籽中提取咖啡因和单宁酸的溶剂优化:分子动力学模拟的启示
瓜拿纳种子含有丰富的生物活性化合物,尤其是咖啡因和单宁酸,这些化合物在食品和制药行业具有重要的应用价值。要从瓜拿纳种子中提取这些宝贵的化合物,需要使用溶剂萃取法。采用分子动力学(MD)模拟研究了瓜拉纳种子提取物与三种不同溶剂(即乙醇、乙酸乙酯和氯化胆碱-甘油作为深共晶溶剂(DES))之间的相互作用。在进行 MD 模拟时,计算了成键和非成键相互作用。液体模拟力场的优化势用于计算这些相互作用。研究通过检测径向分布函数(RDF)和氢键,分别阐明了结构排列和分子间相互作用。RDF 显示咖啡因与乙醇的相互作用最强,因此乙醇是提取咖啡因的最佳溶剂。均方根位移分析有助于深入了解迁移特性,特别是分子在溶剂中的扩散。瓜拉纳种子成分的功能分子在溶剂中的流动性主要表现为亚扩散性。通过比较扩散机制,进一步发现使用乙醇萃取咖啡因和单宁酸比萃取次要化合物更有优势。这种选择性分离使它们在食品工业中得到了重要应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


