Exploring supramolecular C-Br…Br-C interactions as organizing tools in a novel Ni(II)-tetrabromophthalate complex. Crystal structure and solvatochromism studies
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
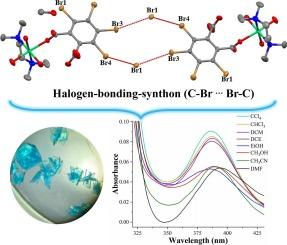
The understanding of the non-covalent halogen…halogen interaction inside coordination complexes has special interest as design tools in crystal engineering and coordination chemistry. In this work, we show the use of the supramolecular C-Br…Br-C interactions as a tool in the crystalline organization of a Ni(II) complex and its solvatochromic properties. To do so, we selected the 3,4,5,6-tetrabromophthalate (TBrPh) ligand where the Br atoms pointing outside to favor their self-assembly through these interactions.
Thus, a novel complex, [Ni(tmeda)(TBrPh)(H2O)3]•CH3OH was synthesized by the reaction between [Ni(tmeda)].(NO3)2 (tmeda = N, N, N’, N’-tetramethylethylenediamine) and K2TBrPh. The Ni(II)-complex was structurally analyzed by single-crystal X-ray diffraction, Hirshfeld surfaces, multiple analytical tools and DFT calculations.
Structural analysis shows that Ni(II)-complex has 1D supramolecular chains based on the cooperativity of intra/inter-molecular charged-assisted hydrogen bonds of type Ni-OH2…O and Csp2-Br…Br-Csp2 interactions into 2D networks. These Br…Br interactions organize the crystal packing in the solid state as is evidenced by the molecular electrostatic potential studies and Plot of Hirsfeld surface.
This Ni(II)-complex displays solvatochromism with a wide range of solvents (alcohols, DMF, CH3CN, chlorinated solvents), and solvent-dependent absorption maxima presents linear behavior as a function of their dielectric constant.
探索超分子 C-Br......Br-C 相互作用作为新型 Ni(II)-tetrabromophthalate 复合物的组织工具。晶体结构和溶解色谱研究
了解配位复合物内部的非共价卤素......卤素相互作用作为晶体工程和配位化学的设计工具具有特殊意义。在这项工作中,我们展示了如何利用超分子 C-Br...Br-C相互作用作为一种工具,来研究镍(II)配合物的晶体结构及其溶解变色特性。为此,我们选择了 3,4,5,6-四溴邻苯二甲酸盐(TBrPh)配体,其中 Br 原子指向外部,通过这些相互作用有利于它们的自组装。因此,[Ni(tmeda)].(NO3)2(tmeda = N,N,N',N'-四甲基乙二胺)和 K2TBrPh 反应合成了一种新型配合物 [Ni(tmeda)(TBrPh)(H2O)3]-CH3OH。通过单晶 X 射线衍射、Hirshfeld 表面、多种分析工具和 DFT 计算,对 Ni(II)-络合物进行了结构分析。结构分析表明,基于分子内/分子间 Ni-OH2...O 型带电辅助氢键和 Csp2-Br...Br-Csp2 型相互作用的协同作用,Ni(II)-络合物具有一维超分子链,形成二维网络。这种 Ni(II)-络合物在多种溶剂(醇类、DMF、CH3CN、氯化溶剂)中都显示出溶解变色作用,并且溶剂依赖性吸收最大值与介电常数呈线性关系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


