Glycerophospholipid metabolic disorders and gender difference of cantharidin-induced hepatotoxicity in rats: Lipidomics and MALDI mass spectrometry imaging analysis
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The hepatotoxicity mechanism of cantharidin (CTD), a major active component of Mylabris was explored based on liver lipidome alterations and spatial distributions in female and male rats using lipidomics and matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI). After oral CTD exposure, the livers of female rats were screened for 104 differential lipids including lysophosphatidylethanolamine(LysoPE)(20:2/0:0) and diacylglycerol(DG)(18:2/22:4), whereas the livers of male rats were screened for 76 differential lipids including fatty acid(FA)(24:6) and DG(18:0/22:4). According to the MALDI-MSI results, female rats exhibited 12 differential lipids with alteration in the abundance and spatial distribution of phosphatylcholine(PC), phosphatidylethanolamine(PE), lysophosphatidylcholine(LysoPC), and LysoPE in the liver lesion area. On the other hand, male rats exhibited 8 differential lipids with changes in the abundance and spatial distribution of PC, PE, and FA in the liver lesion area. The lipidomics- and MALDI-MSI-detected differential lipids strongly disrupted glycerophospholipid metabolism in both female and male rats. Additionally, phosphatidate phosphatase (Lipin1), choline/ethanolamine phosphotransferase 1 (CEPT1), and phosphatidylethanolamine N-methyltransferase (PEMT) were screened to distinguish CTD hepatoxicity in female and male rats. Western blotting analysis demonstrated a significant elevation in Lipin1 expression in female and male rat livers, accompanied by a decrease in PEMT expression. Furthermore, CEPT1 expression increased significantly in female rat livers and decreased significantly in male rat livers. These findings suggested that CTD could disrupt lipid metabolism in a gender-specific manner. Moreover, the combination of lipidomics and MALDI-MSI could offer valuable insights into CTD-induced hepatotoxicity in rats.
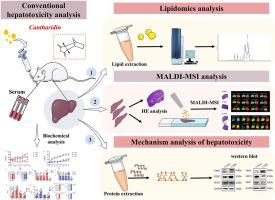
甘油磷脂代谢紊乱与安乃近诱导的大鼠肝毒性的性别差异:脂质组学和 MALDI 质谱成像分析
利用脂质组学和基质辅助激光解吸电离质谱成像(MALDI-MSI)技术,基于雌性和雄性大鼠肝脏脂质体的改变和空间分布,探讨了Mylabris的主要活性成分坎他利定(CTD)的肝毒性机制。口服CTD后,雌性大鼠肝脏中的溶血磷脂酰乙醇胺(LysoPE)(20:2/0:0)和二酰甘油(DG)(18:2/22:4)等104种差异脂质被筛查出来,而雄性大鼠肝脏中的脂肪酸(FA)(24:6)和二酰甘油(DG)(18:0/22:4)等76种差异脂质被筛查出来。根据 MALDI-MSI 的结果,雌性大鼠肝脏病变区域的磷脂酰胆碱(PC)、磷脂酰乙醇胺(PE)、溶血磷脂酰胆碱(LysoPC)和溶血磷脂酰乙醇胺(LysoPE)的丰度和空间分布发生了变化,显示出 12 种差异脂质。另一方面,雄性大鼠表现出 8 种不同的脂质,其中 PC、PE 和 FA 在肝脏病变区域的丰度和空间分布发生了变化。脂质组学和 MALDI-MSI 检测到的差异脂质强烈干扰了雌性和雄性大鼠的甘油磷脂代谢。此外,还对磷脂酰磷酸酶(Lipin1)、胆碱/乙醇胺磷酸转移酶1(CEPT1)和磷脂酰乙醇胺N-甲基转移酶(PEMT)进行了筛选,以区分雌性和雄性大鼠的CTD肝毒性。Western 印迹分析表明,雌性和雄性大鼠肝脏中 Lipin1 的表达显著升高,同时 PEMT 的表达降低。此外,CEPT1 在雌性大鼠肝脏中的表达明显增加,而在雄性大鼠肝脏中则明显减少。这些发现表明,CTD 能以性别特异性的方式破坏脂质代谢。此外,脂质组学和 MALDI-MSI 的结合可为了解 CTD 诱导的大鼠肝毒性提供有价值的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


