Disruption of bioenergetics enhances the radio-sensitivity of patient-derived glioblastoma tumorspheres
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Background
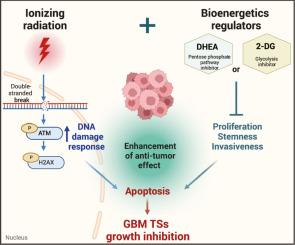
Despite available treatment approaches, including surgical resection along with chemotherapy and radiotherapy, glioblastoma (GBM), the most prevalent primary brain tumor, remains associated with a grim prognosis. Although radiotherapy is central to GBM treatment, its combination with bioenergetics regulators has not been validated in clinical practice. Here, we hypothesized that bioenergetics regulators can enhance the radio-sensitivity of GBM tumorspheres (TSs).
Methods
Gene expression profiles of GBM patient-derived TSs were obtained through microarray and RNA-seq. In vitro treatment efficacy was assessed using clonogenic assay, 3D invasion assay, neurosphere formation assay, and flow cytometry. Protein expression was measured via western blot, and γH2AX foci were detected via immunofluorescence. In vivo efficacy was confirmed in an orthotopic xenograft model.
Results
Based on radiation response-associated gene expression, GBM TSs were classified into high- or low-radioresistant groups. Among the five bioenergetics regulators, the pentose phosphate pathway inhibitor DHEA and the glycolysis inhibitor 2-DG notably enhanced the efficacy of ionizing radiation (IR) efficacy in vitro, reducing the survival fraction, stemness, and invasiveness in high- and low-radioresistant TSs. Combination with 2-DG further stimulated IR-induced DNA damage response and apoptosis in low-radioresistant GBM TSs. RNA-seq analysis revealed a downregulation of bioenergetics- and cell cycle-associated genes, whereas extracellular matrix- and cell adhesion-associated genes were enhanced by combined IR and 2-DG treatment. This therapeutic regimen extended survival and diminished tumor size in mouse xenograft models.
Conclusions
Our data suggest that combination with bioenergetics regulator 2-DG enhances the radio-sensitivity of GBM TSs, highlighting the clinical potential of this combined regimen.
破坏生物能增强源自患者的胶质母细胞瘤肿瘤球的放射敏感性
背景:尽管现有的治疗方法包括手术切除、化疗和放疗,但胶质母细胞瘤(GBM)这种最常见的原发性脑肿瘤的预后仍然很差。尽管放疗是 GBM 治疗的核心,但其与生物能调节剂的结合尚未在临床实践中得到验证。在此,我们假设生物能调节剂能增强GBM瘤球(TSs)的放射敏感性:方法:通过芯片和 RNA-seq 获得 GBM 患者来源 TS 的基因表达谱。采用克隆形成试验、三维侵袭试验、神经球形成试验和流式细胞术评估体外治疗效果。蛋白表达通过 Western 印迹进行测量,γH2AX 病灶通过免疫荧光进行检测。结果:结果:根据辐射反应相关基因的表达,GBM TSs 被分为高耐受辐射组和低耐受辐射组。在五种生物能调节剂中,磷酸戊糖途径抑制剂 DHEA 和糖酵解抑制剂 2-DG 显著增强了电离辐射(IR)的体外疗效,降低了高耐受性和低耐受性 TS 的存活率、干性和侵袭性。与 2-DG 联用可进一步刺激红外诱导的 DNA 损伤反应和低耐受性 GBM TS 的细胞凋亡。RNA-seq分析显示生物能和细胞周期相关基因下调,而细胞外基质和细胞粘附相关基因在IR和2-DG联合治疗下增强。这种治疗方案延长了小鼠异种移植模型的生存期并缩小了肿瘤大小:我们的数据表明,与生物能调节剂 2-DG 联合使用可增强 GBM TSs 的放射敏感性,凸显了这种联合疗法的临床潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


