In Vitro and Vivo Experiments Revealing Astragalin Inhibited Lung Adenocarcinoma Development via LINC00582/miR-140-3P/PDPK1
Abstract
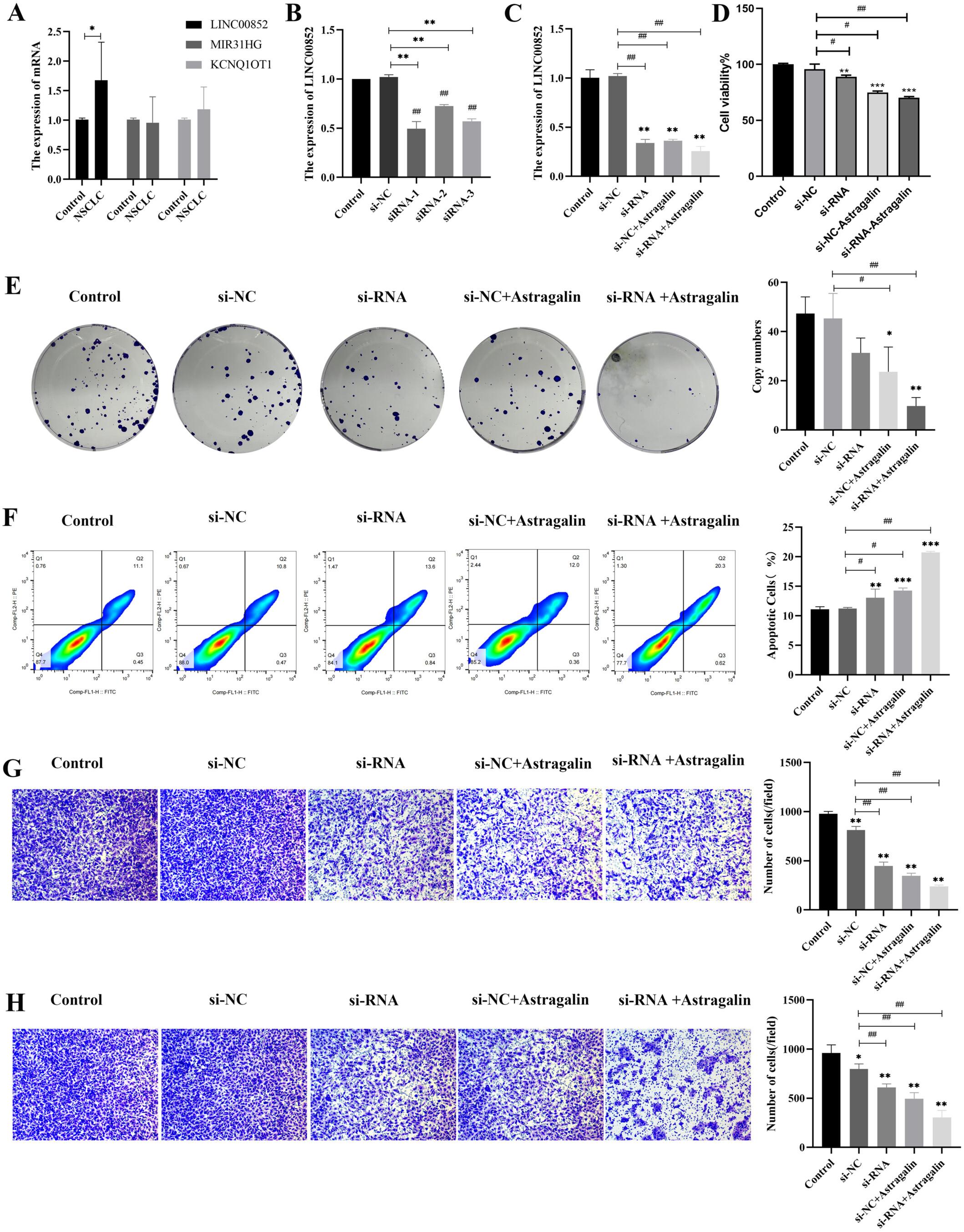
This study aimed to explore the mechanism of the development of lung adenocarcinoma (LUAD) treated by astragalin. Transcriptome sequencing was performed to obtain the gene profile of LUAD treated by astragalin. Combining with bioinformatics analysis including differential gene screening, function enrichment analysis (gene ontology and KEGG), and ceRNA construction, we obtained the novel mechanism of lncRNA mediated miRNA/mRNA axis. Then, the cell experiments were performed to examine the role of lncRNA in cell proliferation, migration and invasion, and apoptosis for LUAD treated with astragalin. Moreover, the tumor formation in nude mice was carried out to detect the ceRNA mechanism in LUAD treated by astragalin in vivo. The lncRNA mediated ceRNA network was obtained, that is, LINC00852 LINC00582/miR-140-3p/PDPK1 played an important role in LUAD treated by astragalin. Function experiments indicated that si-LINC00852 inhibited LUAD cell proliferation, migration and invasion, and promoted cell apoptosis via miR-140-3p/PDPK1 (p < 0.05, p < 0.01). The animal experiments further confirmed that si-LINC00852 inhibited tumor growth through miR-140-3p/PDPK1 in vivo. Conversely, this study provides comprehensive insights into the diagnostic and therapeutic implications of LINC00582 in LUAD, LINC00582 mediated miR-140-3p/PDPK1 axis was the novel drug target of astragalin for treating LUAD.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


