Inducible and reversible SOD2 knockdown in mouse skeletal muscle drives impaired pyruvate oxidation and reduced metabolic flexibility
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Introduction
Skeletal muscle mitochondrial dysfunction is a key characteristic of aging muscle and contributes to age related diseases such as sarcopenia, frailty, and type 2 diabetes. Mitochondrial oxidative stress has been implicated as a driving factor in these age-related diseases, however whether it is a cause, or a consequence of mitochondrial dysfunction remains to be determined. The development of flexible genetic models is an important tool to test the mechanistic role of mitochondrial oxidative stress on skeletal muscle metabolic dysfunction. We characterize a new model of inducible and reversible mitochondrial redox stress using a tetracycline controlled skeletal muscle specific short hairpin RNA targeted to superoxide dismutase 2 (iSOD2).
Methods: iSOD2 KD and control (CON) animals were administered doxycycline for 3- or 12- weeks and followed for up to 24 weeks and mitochondrial respiration and muscle contraction were measured to define the time course of SOD2 KD and muscle functional changes and recovery.
Results
Maximum knockdown of SOD2 protein occurred by 6 weeks and recovered by 24 weeks after DOX treatment. Mitochondrial aconitase activity and maximum mitochondrial respiration declined in KD muscle by 12 weeks and recovered by 24 weeks. There were no significant differences in antioxidant or mitochondrial biogenesis genes between groups. Twelve-week KD showed a small, but significant decrease in muscle fatigue resistance. The primary phenotype was reduced metabolic flexibility characterized by impaired pyruvate driven respiration when other substrates are present. The pyruvate dehydrogenase kinase inhibitor dichloroacetate partially restored pyruvate driven respiration, while the thiol reductant DTT did not.
Conclusion
We use a model of inducible and reversible skeletal muscle SOD2 knockdown to demonstrate that elevated matrix superoxide reversibly impairs mitochondrial substrate flexibility characterized by impaired pyruvate oxidation. Despite the bioenergetic effect, the limited change in gene expression suggests that the elevated redox stress in this model is confined to the mitochondrial matrix.
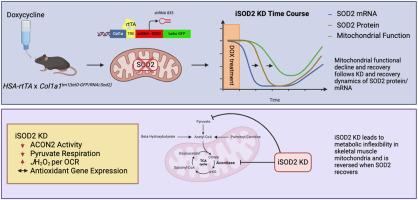
小鼠骨骼肌中诱导性和可逆性 SOD2 基因敲除导致丙酮酸氧化功能受损和代谢灵活性降低。
导言:骨骼肌线粒体功能障碍是肌肉老化的一个主要特征,也是肌肉疏松症、虚弱症和 2 型糖尿病等老年相关疾病的诱因。线粒体氧化应激被认为是这些与年龄有关的疾病的驱动因素,但它是线粒体功能障碍的原因还是结果仍有待确定。开发灵活的遗传模型是检验线粒体氧化应激对骨骼肌代谢功能障碍的机理作用的重要工具。我们利用四环素控制的骨骼肌特异性短发夹RNA靶向超氧化物歧化酶2(iSOD2),鉴定了一种诱导性和可逆性线粒体氧化还原应激的新模型。方法:给iSOD2 KD和对照组(CON)动物注射强力霉素3周或12周,随访24周,测量线粒体呼吸和肌肉收缩,以确定SOD2 KD和肌肉功能变化及恢复的时间过程:结果:SOD2蛋白的最大敲除发生在DOX治疗后6周,并在24周前恢复。KD 肌肉的线粒体丙酮酸酶活性和线粒体最大呼吸量在 12 周前下降,在 24 周前恢复。不同组间的抗氧化或线粒体生物生成基因没有明显差异。为期 12 周的 KD 显示肌肉抗疲劳能力有小幅但显著的下降。主要表型是代谢灵活性降低,其特点是当存在其他底物时,丙酮酸驱动的呼吸作用受损。丙酮酸脱氢酶激酶抑制剂二氯乙酸可部分恢复丙酮酸驱动的呼吸,而硫醇还原剂DTT则不能:我们利用诱导性和可逆性骨骼肌 SOD2 基因敲除模型证明,基质超氧化物的升高会可逆地损害线粒体底物的灵活性,其特征是丙酮酸氧化能力受损。尽管存在生物能量效应,但基因表达的有限变化表明,该模型中升高的氧化还原压力仅限于线粒体基质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


