Cr-doped NiFe sulfides nanoplate array: Highly efficient and robust bifunctional electrocatalyst for the overall water splitting and seawater electrolysis
IF 9.4
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
To replace precious metals and reduce production costs for large-scale hydrogen production, developing stable, high-performance transition metal electrocatalysts that can be used in a wide range of environments is desirable yet challenging. Herein, a self-supported hybrid catalyst (NiFeCrSx/NF) with high electrocatalytic activity was designed and constructed using conductive nickel foam as a substrate via manipulation of the cation doping ratio of transition metal compounds. Due to the strong coupling synergy between the metal sulfides NiS2, Fe9S11, and Cr2S3, as well as their interaction with the conductive nickel foam (NF), the energy barrier for catalytic reactions is reduced, and the charge transfer rate is enhanced. This significantly improves the hydrogen evolution reaction (HER) performance of NiFeCrSx/NF, achieving a current density of 10 mA cm−2 with an overpotential of just 66 mV. Furthermore, doping with chromium generates different valence states of Cr during the catalytic process, which can synergize with the high-valent Fe and Ni, promoting the formation of oxygen vacancies and enriching the active sites for the oxygen evolution reaction (OER). Consequently, at a current density of 10 mA cm−2 in 1.0 M KOH, the overpotential for OER is only 223 mV for NiFeCrSx/NF. Additionally, the in situ grown of self-supporting nanoflower structure on NiFe-LDH not only provides a large catalytic surface area but also facilitates electrolyte penetration during the catalytic process, endowing NiFeCrSx/NF with high long-term stability. When used as a bifunctional catalyst for overall water splitting, the NiFeCrSx/NF||NiFeCrSx/NF electrolyzer requires only 1.29 V to deliver a current density of 10 mA cm−2. Simultaneously, Cr doping protects the Fe sites by maintaining stable valence states, ensuring high performance and stability of NiFeCrSx/NF, even when it is utilized for seawater splitting. This strategy offers novel concepts for creating catalysts based on non-precious metals that can be utilized in various application scenarios.
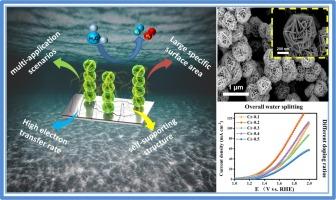
掺杂铬的镍铁硫化物纳米板阵列:用于整体水分离和海水电解的高效、稳健的双功能电催化剂。
为了取代贵金属并降低大规模制氢的生产成本,开发可在多种环境中使用的稳定、高性能过渡金属电催化剂是非常理想的,但也是极具挑战性的。在此,通过操纵过渡金属化合物的阳离子掺杂比例,设计并构建了一种以导电泡沫镍为基底的具有高电催化活性的自支撑混合催化剂(NiFeCrSx/NF)。由于金属硫化物 NiS2、Fe9S11 和 Cr2S3 之间的强耦合协同作用,以及它们与导电泡沫镍(NF)之间的相互作用,降低了催化反应的能量势垒,提高了电荷转移速率。这大大提高了 NiFeCrSx/NF 的氢进化反应(HER)性能,使其电流密度达到 10 mA cm-2,过电位仅为 66 mV。此外,在催化过程中,铬的掺杂会产生不同价态的铬,与高价的铁和镍协同作用,促进氧空位的形成,丰富氧进化反应(OER)的活性位点。因此,在 1.0 M KOH 中,当电流密度为 10 mA cm-2 时,NiFeCrSx/NF 的氧进化反应过电位仅为 223 mV。此外,NiFe-LDH 上原位生长的自支撑纳米花结构不仅提供了较大的催化表面积,还有利于催化过程中电解质的渗透,从而使 NiFeCrSx/NF 具有较高的长期稳定性。当用作整体水分离的双功能催化剂时,NiFeCrSx/NF||NiFeCrSx/NF 电解槽只需要 1.29 V 的电压就能提供 10 mA cm-2 的电流密度。同时,铬的掺杂通过保持稳定的价态来保护铁的位点,从而确保了 NiFeCrSx/NF 的高性能和稳定性,即使将其用于海水分离也是如此。这种策略为创造基于非贵金属的催化剂提供了新的概念,这种催化剂可用于各种应用场合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


