Targeting bacterial efflux pump effectively enhances the efficacy of Ru-based antibacterial agents against Gram-negative pathogen
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The rise of antibiotic resistance has posed a great threat to human's life, thus develop novel antibacterial agents is urgently needed. It worthies to noted that Ru-based antibacterial agents often showed robust potency against Gram-positive pathogens, disrupted bacterial membrane and avoided bacterial resistance, making they promising antibiotic candidates. However, they are generally less active when applied to negative pathogens. To address this problem, a Ru-based metalloantibiotic (RuN) modified with a nitrothiophene moiety, which can target bacterial efflux pump, was designed and evaluated in this work. A series of assays demonstrated that RuN not only fully retained the advantages of Ru-based agents, such as destroyed bacterial membrane and induced reactive oxygen species production, but also can targeted bacterial efflux pumps. Of course, these properties make it effective in killing both Gram-positive and negative pathogens, its MIC values against Staphylococcus aureus and Escherichia coli lies at 3.125 and 6.25 μg/mL, respectively. Importantly, RuN also showed low toxicity and has robust anti-infective potency in two animal infection models. Together, our results paved an alternative way to enhance the anti-infective efficacy of Ru-based agents against resistant negative bacteria.
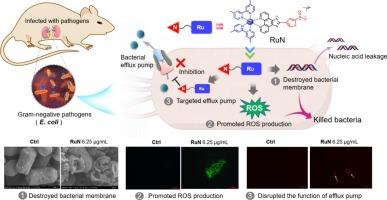
以细菌外排泵为靶点可有效提高 Ru 类抗菌剂对革兰氏阴性病原体的疗效。
抗生素耐药性的增加对人类生命构成了巨大威胁,因此开发新型抗菌剂迫在眉睫。值得注意的是,基于 Ru 的抗菌剂通常对革兰氏阳性病原体表现出强大的效力,能破坏细菌膜并避免细菌产生抗药性,因此是很有前途的抗生素候选物。然而,当它们用于阴性病原体时,活性通常较低。为解决这一问题,本研究设计并评估了一种由硝基噻吩分子修饰的 Ru 基金属抗生素(RuN),它可以靶向细菌外排泵。一系列检测结果表明,RuN 不仅完全保留了 Ru 类药物的优点,如破坏细菌膜和诱导活性氧产生,而且还能靶向细菌外排泵。当然,这些特性使其能有效杀死革兰氏阳性和阴性病原体,其对金黄色葡萄球菌和大肠杆菌的 MIC 值分别为 3.125 和 6.25 μg/mL。重要的是,RuN 在两种动物感染模型中也显示出低毒性和强大的抗感染效力。总之,我们的研究结果为提高基于 Ru 的制剂对耐药阴性细菌的抗感染效力铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


