Copper(I)-Catalyzed Asymmetric Nucleophilic Addition to Aldehydes with Skipped Enynes.
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The development of sustainable and novel strategies for constructing complex chiral molecules with versatile transformation potential is a long-term pursuit in the chemistry community. We report a copper(I)-catalyzed enyne addition to aldehydes under proton-transfer conditions, unlike previous examples which were limited to the use of preformed reactive nucleophiles containing allylic heteroatoms or electron-withdrawing groups. This protocol provides an efficient platform for installing chiral allylic alcohol moieties with a broad substrate scope and high regio-, stereo-, and enantioselectivity.
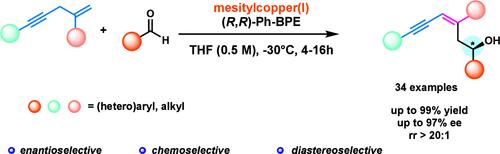
铜(I)催化的醛与跳炔的不对称亲核加成。
开发可持续的新策略来构建具有多功能转化潜力的复杂手性分子是化学界的一项长期追求。我们报告了在质子转移条件下铜(I)催化的烯炔与醛的加成反应,这与之前的例子不同,之前的例子仅限于使用含有烯丙基杂原子或电子撤回基团的预成反应性亲核物。该方案为安装手性烯丙基醇分子提供了一个高效平台,具有广泛的底物范围和高区域、立体和对映选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


