L-Asparaginase from Lachancea Thermotolerans: Effect of Lys99Ala on Enzyme Performance and in vitro Antileukemic Efficacy
Abstract
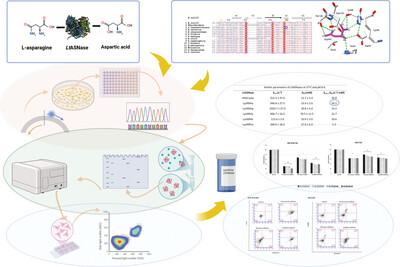
L-asparaginases (EC 3.5.1.1) are amidohydrolase enzymes that predominantly catalyze conversion of L-asparagine to L-aspartic acid and ammonia. In addition, some exhibit secondary L-glutaminase activity. Escherichia coli and Erwinia chrysanthemi L-asparaginases are widely used in the pharmaceutical industry to produce therapeutically important compounds. In the therapeutic use of enzymes, bacterial L-asparaginases can trigger immune responses, leading to a high rate of adverse effects that diminish the effectiveness of the treatment. This situation has forced scientists to search for promising L-asparaginases from new sources. Yeast L-asparaginases could be useful in reducing toxicity and enhancing efficacy but they have been poorly studied to date. Here, we characterized the yeast Lachancea thermotolerans L-asparaginase (LtASNase) purified by affinity chromatography. It has a specific activity of 313.8 U/mg and a high kcat value (312.4 s). We demonstrated through a semi-rational design that the mutations of Lys99 show varying effects on catalytic activity, with the Lys99Ala mutant increasing specific activity 3.3-fold. Furthermore, the in vitro antileukemic activity of the non-formulated form of Lys99Ala LtASNase was evaluated against SUP-B15 and REH cell lines. The results demonstrated that LtASNase exhibits significant antileukemic potential, comparable to commercial type II bacterial enzymes. The understanding of the mutant L-asparaginases examined in this study will significantly contribute to the development of new and more effective yeast-derived asparaginases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


