LncRNA MYOSLID contributes to PH via targeting BMPR2 signaling in pulmonary artery smooth muscle cell
IF 3.5
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background/objective
The pathogenesis and vascular remodeling during pulmonary hypertension (PH) have been associated with dysregulation of bone morphogenetic protein receptor type 2 (BMPR2) and transforming growth factor-β (TGF-β) signaling in pulmonary artery smooth muscle cells (PASMCs). Evidence suggests that the human-specific lncRNA MYOSLID is a transcriptional target of the TGF-β/SMAD pathway. In this study, we investigated the involvement of MYOSLID in the pathogenesis of PH.
Methods
Lung tissues from PH patients and rat PH models were analyzed to assess clinical relevance. RNA-Seq was performed to identify target genes. Pulmonary artery smooth muscle cells (PASMCs) were used to evaluate function and underlying mechanisms.
Results
RNA-Seq analysis of PASMCs stimulated by TGF-β1 revealed significantly dysregulated lncRNAs. MYOSLID expression was markedly elevated in lung tissues from PH patients and in PASMCs stimulated with TGF-β1. Mechanistically, loss of MYOSLID inhibited the TGF-β pathway by reducing SMAD2/3 PHosphorylation and activated the BMPR2 pathway by enhancing SMAD1/5/9 phosphorylation and increasing ID genes expression in PASMCs. DAZAP2, a target gene of MYOSLID, functions as an inhibitor of BMPR2 signaling. Moreover, DAZAP2 expression was significantly elevated in lung tissues from PH patients and rat PH models. Functionally, knockdown of MYOSLID and DAZAP2 reduced proliferation, migration, and apoptosis resistance in PASMCs.
Conclusion
The activation of the MYOSLID-DAZAP2-BMPR2 axis contributes to pulmonary vascular remodeling, and targeting MYOSLID and DAZAP2 may represent novel therapeutic strategies for PH treatment.
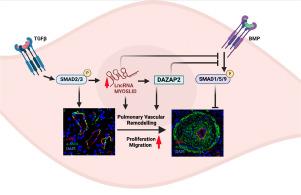
LncRNA MYOSLID通过靶向肺动脉平滑肌细胞中的BMPR2信号对PH做出了贡献。
背景/目的:肺动脉高压(pH)的发病机制和血管重塑与肺动脉平滑肌细胞(PASMCs)中骨形态发生蛋白受体2型(BMPR2)和转化生长因子-β(TGF-β)信号传导失调有关。有证据表明,人类特异性 lncRNA MYOSLID 是 TGF-β/SMAD 通路的转录靶标。在这项研究中,我们探讨了MYOSLID参与PH发病机制的情况:方法:分析 PH 患者和大鼠 PH 模型的肺组织以评估临床相关性。进行RNA-Seq分析以确定靶基因。肺动脉平滑肌细胞(PASMCs)用于评估功能和潜在机制:结果:对受到 TGF-β1 刺激的肺动脉平滑肌细胞进行 RNA-Seq 分析,发现了显著失调的 lncRNAs。在PH患者的肺组织和受TGF-β1刺激的PASMCs中,MYOSLID的表达明显升高。从机制上讲,MYOSLID的缺失通过降低SMAD2/3 pH磷酸化抑制了TGF-β通路,并通过增强SMAD1/5/9磷酸化和增加PASMCs中ID基因的表达激活了BMPR2通路。MYOSLID的靶基因DAZAP2是BMPR2信号传导的抑制剂。此外,DAZAP2在PH患者和大鼠PH模型肺组织中的表达明显升高。从功能上讲,敲除 MYOSLID 和 DAZAP2 可减少 PASMCs 的增殖、迁移和抗凋亡能力:结论:MYOSLID-DAZAP2-BMPR2 轴的激活有助于肺血管重塑,靶向 MYOSLID 和 DAZAP2 可能是治疗 PH 的新型治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Vascular pharmacology
医学-药学
CiteScore
6.60
自引率
2.50%
发文量
153
审稿时长
31 days
期刊介绍:
Vascular Pharmacology publishes papers, which contains results of all aspects of biology and pharmacology of the vascular system.
Papers are encouraged in basic, translational and clinical aspects of Vascular Biology and Pharmacology, utilizing approaches ranging from molecular biology to integrative physiology. All papers are in English.
The Journal publishes review articles which include vascular aspects of thrombosis, inflammation, cell signalling, atherosclerosis, and lipid metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


