Identification of the central tolerance checkpoint for autoreactive proteinase 3+ B cells in human bone marrow
IF 7.9
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Major target antigens of ANCA-associated vasculitis (AAV) are myeloperoxidase (MPO) and proteinase 3 (PR3). High-affinity MPO- and PR3-ANCA immunoglobulins are produced by antigen-experienced, class-switched autoreactive B cells. To prevent autoreactivity, B cells are subjected to several self-tolerance checkpoints, from the early immature stages in the bone marrow (BM), collectively called “central tolerance”, to late mature stages, collectively called “peripheral tolerance”; the latter was recently elucidated for autoreactive PR3+ B cells.
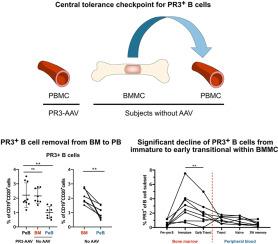
Here we investigated central tolerance controlling immature PR3+B cells in the BM before their migration into the periphery as transitional B cells. We applied an established flow cytometry-based method using labeled recombinant PR3 (rPR3) to identify the PR3+B cells to compare the phenotype of PR3+B cells in paired samples of BM mononuclear cells (BMMC) and peripheral blood mononuclear cells (PBMC) of non-vasculitis controls (No-AAV), and PBMC of patients with PR3-ANCA-associated vasculitis (PR3-AAV).
We observed that the proportion of PR3+B cells within BMMC was higher (median [IQR]; 1.98 % [1.77–2.75]) than within PBMC of No-AAV (0.9 % [0.63–1.44], p < 0.01 by paired comparison) and similar to their proportion within PBMC of patients with PR3-AAV (1.82 % [1.66–3.21]; p > 0.05). Within CD24++CD38++ B cells, the subset of B cell migrating from BM to the periphery, BMMC contained a greater proportion of PR3+B cells as compared to PBMC in No-AAV (3.35 % [1.99–4.92] versus 1.23 % [0.62–1.55], p < 0.01), showing different surface markers of maturation (i.e. higher proportion of CD27−CD10+ and lower expression of CD21, IgD, IgM). Importantly, we observed a significant decline of the PR3+ fraction from the immature subset (IgD−IgM+; 2.80 % [1.23–4.02]) to the early transitional subset (IgD+IgM+; 1.76 % [0.96–2.68], p < 0.01) in BMMC, while no significant reduction was observed between the early transitional of BMMC and the transitional compartment of PBMC in No-AAV (1.26 % [0.62–1.56], p > 0.05).
In conclusion, to prevent PR3-related autoimmunity, autoreactive PR3+B cells pass a stringent selection in the BM, and their removal by central tolerance checkpoint activity occurs mainly between T1-like/immature to T2-like/early transitional B cells of BMMC.
确定人类骨髓中自反应蛋白酶 3+ B 细胞的中央耐受检查点。
ANCA 相关性血管炎(AAV)的主要靶抗原是髓过氧化物酶(MPO)和蛋白酶 3(PR3)。高亲和力 MPO 和 PR3-ANCA 免疫球蛋白是由抗原经验丰富的类开关自反应 B 细胞产生的。为了防止自反应性,B 细胞要经过几个自我耐受检查点,从骨髓(BM)中的早期不成熟阶段(统称为 "中心耐受")到晚期成熟阶段(统称为 "外周耐受");最近对自反应性 PR3+ B 细胞阐明了后者。在此,我们研究了在未成熟的 PR3+B 细胞作为过渡性 B 细胞迁移到外周之前,控制其在 BM 中的中心耐受性。我们采用了一种基于流式细胞术的方法,用标记的重组 PR3(rPR3)来识别 PR3+B 细胞,比较了非脉管炎对照组(No-AAV)和 PR3-ANCA 相关性脉管炎患者(PR3-AAV)的成对样本的 BM 单核细胞(BMMC)和外周血单核细胞(PBMC)中 PR3+B 细胞的表型。我们观察到,BMMC 中 PR3+B 细胞的比例(中位数 [IQR];1.98 % [1.77-2.75])高于 No-AAV 患者的 PBMC(0.9 % [0.63-1.44],P 0.05)。在 CD24++CD38++ B 细胞(从 BM 迁移到外周的 B 细胞亚群)中,与 No-AAV 的 PBMC 相比,BMMC 含有更大比例的 PR3+B 细胞(3.35 % [1.99-4.92] 对 1.23 % [0.62-1.55],p -CD10+,CD21、IgD、IgM 表达较低)。重要的是,我们观察到 PR3+ 部分从未成熟亚群(IgD-IgM+;2.80 % [1.23-4.02])显著下降到早期过渡亚群(IgD+IgM+;1.76 % [0.96-2.68],p 0.05)。总之,为防止与 PR3 相关的自身免疫,自身反应性 PR3+B 细胞在 BM 中经过了严格的筛选,它们主要在 BMMC 的 T1 样/未成熟到 T2 样/早期过渡 B 细胞之间被中枢耐受检查点活动清除。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of autoimmunity
医学-免疫学
CiteScore
27.90
自引率
1.60%
发文量
117
审稿时长
17 days
期刊介绍:
The Journal of Autoimmunity serves as the primary publication for research on various facets of autoimmunity. These include topics such as the mechanism of self-recognition, regulation of autoimmune responses, experimental autoimmune diseases, diagnostic tests for autoantibodies, as well as the epidemiology, pathophysiology, and treatment of autoimmune diseases. While the journal covers a wide range of subjects, it emphasizes papers exploring the genetic, molecular biology, and cellular aspects of the field.
The Journal of Translational Autoimmunity, on the other hand, is a subsidiary journal of the Journal of Autoimmunity. It focuses specifically on translating scientific discoveries in autoimmunity into clinical applications and practical solutions. By highlighting research that bridges the gap between basic science and clinical practice, the Journal of Translational Autoimmunity aims to advance the understanding and treatment of autoimmune diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


