Pre-lithiation synergized with magnesiothermic reduction to enhance the performance of SiO anode for advanced lithium-ion batteries
IF 9.4
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Due to its high theoretical specific capacity, micron-sized silicon monoxide (SiO) is regarded as one of the most competitive anode materials for lithium-ion batteries with high specific energy density. However, originating from the low initial Coulombic efficiency (ICE) and large volume expansion, its large-scale application is seriously hindered. Herein, an easy-to-implement solid-state pre-lithiation method synergized with the magnesiothermic reduction process was performed to enhance the ICE of SiO and a common bimetallic hydride was used as a prelithiation reagent. Moreover, the effects of different pre-lithiation reagent amounts on the physical and electrochemical properties of SiOx are investigated. Notably, the SiOx-LA@C composite anchored by in-situ generated LiAl(SiO3)2 shows a more stable microstructure and excellent electrochemical properties, which delivers an ultrahigh ICE of 89.4 % and an excellent initial capacity of 1864.4 mAh g−1. Furthermore, the full cells were successfully assembled by using the prepared anodes, which exhibit relatively stable cycle performance over 150 cycles. This work suggests a safe and feasible route to enhance the ICE of SiOx for the applicable SiO-based anode materials.
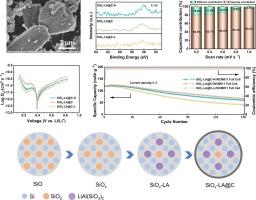
预硫化与镁热还原协同提高了先进锂离子电池的氧化硅负极性能。
由于理论比容量高,微米级一氧化硅(SiO)被认为是高比能量密度锂离子电池最具竞争力的负极材料之一。然而,由于初始库仑效率(ICE)低和体积膨胀大,其大规模应用受到严重阻碍。本文采用一种易于实现的固态预硫化方法,与镁热还原过程协同提高氧化硅的ICE,并使用一种常见的双金属氢化物作为预硫化试剂。此外,还研究了不同预硫化试剂用量对 SiOx 物理和电化学性质的影响。值得注意的是,由原位生成的 LiAl(SiO3)2锚定的 SiOx-LA@C 复合材料显示出更稳定的微观结构和优异的电化学性能,可实现 89.4 % 的超高 ICE 和 1864.4 mAh g-1 的出色初始容量。此外,使用制备的阳极还成功组装了完整的电池,并在 150 次循环中表现出相对稳定的循环性能。这项工作为适用的氧化硅基阳极材料提高氧化硅的 ICE 提出了一条安全可行的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


