Exploring divalent metal ion coordination. Unraveling binding modes in Staphylococcus aureus MntH fragments
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Metal ion coordination is crucial in bacterial metabolism, while divalent metal ions serve as essential cofactors for various enzymes involved in cellular processes. Therefore, bacteria have developed sophisticated regulatory mechanisms to maintain metal homeostasis. These involve protein interactions for metal ion uptake, efflux, intracellular transport, and storage. Staphylococcus aureus, a member of the commensal flora, colonizes the anterior nares and skin harmlessly but can cause severe illness. MntH transporter is responsible for acquiring divalent metal ions necessary for metabolic functions and virulence. It is a 450-amino-acid protein analogous to Nramp1 (Natural Resistance-Associated Macrophage Protein 1) in mammals. Herein, the coordination modes of copper(II), iron(II), and zinc(II) ions with select fragments of the MntH were established employing potentiometry, mass spectrometry, and spectroscopic methods. Four model peptides, MNNKRHSTNE–NH2, Ac-KFDHRSS–NH2, Ac-IMPHNLYLHSSI–NH2, and Ac-YSRHNNEE–NH2, were chosen for their metal-binding capabilities and examined to determine their coordination properties and preferences. Our findings suggest that under physiological pH conditions, the N-terminal fragment of MntH demonstrates the highest thermodynamic stability with copper(II) and iron(II) ions. Furthermore, a comparison with other peptides from the S. aureus FeoB transporter indicates different binding affinities.
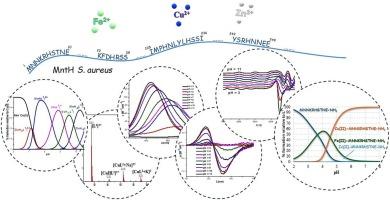
探索二价金属离子的配位。揭示金黄色葡萄球菌 MntH 片段的结合模式。
金属离子配位在细菌的新陈代谢中至关重要,而二价金属离子则是参与细胞过程的各种酶所必需的辅助因子。因此,细菌开发了复杂的调节机制来维持金属平衡。这些机制涉及金属离子摄取、外流、细胞内运输和储存的蛋白质相互作用。金黄色葡萄球菌是共生菌群中的一员,定植于前鼻孔和皮肤,对人体无害,但可导致严重疾病。MntH 转运体负责获取代谢功能和毒力所需的二价金属离子。它是一种含有 450 个氨基酸的蛋白质,类似于哺乳动物中的 Nramp1(自然抗性相关巨噬细胞蛋白 1)。本文采用电位测定法、质谱法和光谱法确定了铜(II)、铁(II)和锌(II)离子与 MntH 某些片段的配位模式。我们选择了四种具有金属结合能力的模型肽:MNNKRHSTNE-NH2、Ac-KFDHRSS-NH2、Ac-IMPHNLYLHSSI-NH2 和 Ac-YSRHNEE-NH2,并对它们进行了研究,以确定它们的配位特性和偏好。我们的研究结果表明,在生理 pH 值条件下,MntH 的 N 端片段与铜(II)和铁(II)离子的热力学稳定性最高。此外,与来自金黄色葡萄球菌 FeoB 转运体的其他肽的比较表明,它们具有不同的结合亲和力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


